Chapter – 4
Carbon and It’s Compounds
Carbon is the third most important element after oxygen and hydrocarbon, for the existence of life on the earth. The Earth crust has only 0.02% carbon which is present in the form of minerals (like carbonates, hydrogen-carbonates, coal, petroleum, etc.) and the atmosphere has 0.03 % of carbon dioxide.
Fuels (like wood, kerosene, coal, LPG, CNG, petrol, etc.) clothing material (like cotton, nylon, polyester, etc..) paper, rubber, plastics, leather, drugs and dyes are all made up of carbon.
Covalent Bonding in Carbon Compounds
The bond formed by sharing an electron pair between the atoms (either same or different atoms) are known as covalent bonds.
Atomic number of carbon (C) is 6. So, its electronic configuration = 2, 4 ( 2 electrons in K-Shell & 4 electrons in L- Shell).
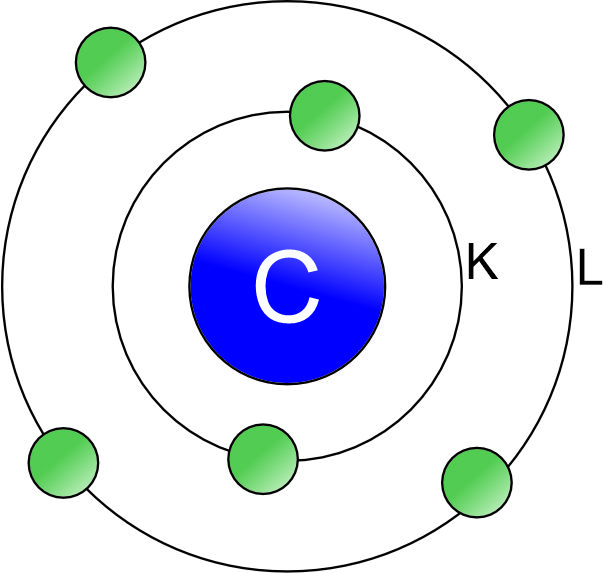
Thus, there are 4 electrons in its outermost shell and its octet can be completed by the following two ways:
- It could gain 4 electrons and form C4- anion. But for a nucleus having 6 electrons, it would be difficult to hold on 10 electrons, i.e., 4 extra electrons.
- It could loss 4 electrons and form C4+ cation. But a large amount of energy is required to remove 4 electrons leaving behind a carbon cation with 6 protons in its nucleus holding on just two electrons together, which is not possible
Thus, carbon atom cannot enter into bonding both by either losing its valence electrons or by gaining 4 more electrons. In order to overcome these problems, carbon shares its valence electrons with other atoms of carbon or with atoms of other elements. These shared electrons belong to the outermost shells of both atoms and in this way, both atoms attain the nearest noble gas configuration. This type of bonding is called covalent bonding.
Compounds having covalent bonds are called covalent compounds. These are generally poor conductor of electricity.
Types of Covalent Bonds:
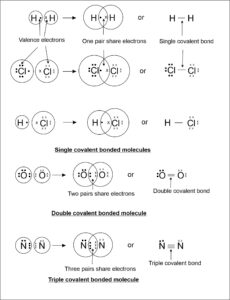
- Single Covalent Bond: The covalent bond formed by sharing one pair of electrons between two atoms is called single covalent bond. For example, covalent bond formed in H2, F2, Cl2, Br2, I2 , HF, HCl, HBr, etc.
- Double Covalent Bond: The covalent bond formed by sharing two pairs of electrons between two atoms is called double covalent bond. For example, covalent bond formed in O2 etc.
- Triple Covalent Bond: The covalent bond formed by sharing three pairs of electrons between two atoms is called triple covalent bond. For example, covalent bond formed in N2 etc.
Other Examples of Covalent Bonding
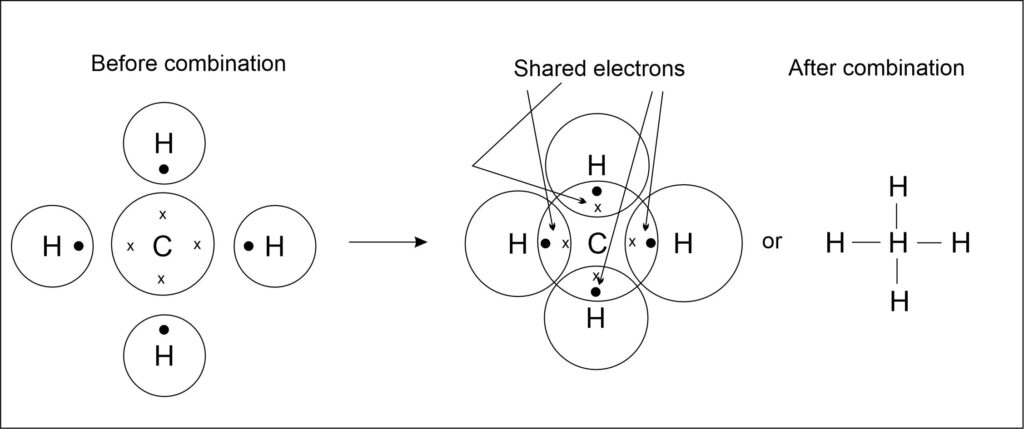
1. Formation of Methane (CH4) molecule
In the formation of methane molecule, one carbon atom shares its 4 electrons with four hydrogen atoms (one electron of each hydrogen atom). It shows carbon is tetravalent because it possesses 4 valence electrons and hydrogen is monovalent because it has only 1 valence electron.
2. Formation of water (H2O) molecule
Atomic number of O = 8, Electronic configuration = 2, 6

To attain the stable electronic configuration of the nearest noble gas, hydrogen needs 1 electron and oxygen needs 2 electrons. So, the oxygen atoms share 2 electrons with two hydrogen atoms ( one electron with each hydrogen atom) such that hydrogen acquires a doublet configuration and oxygen an octet, resulting in the formation of two single covalent bonds.
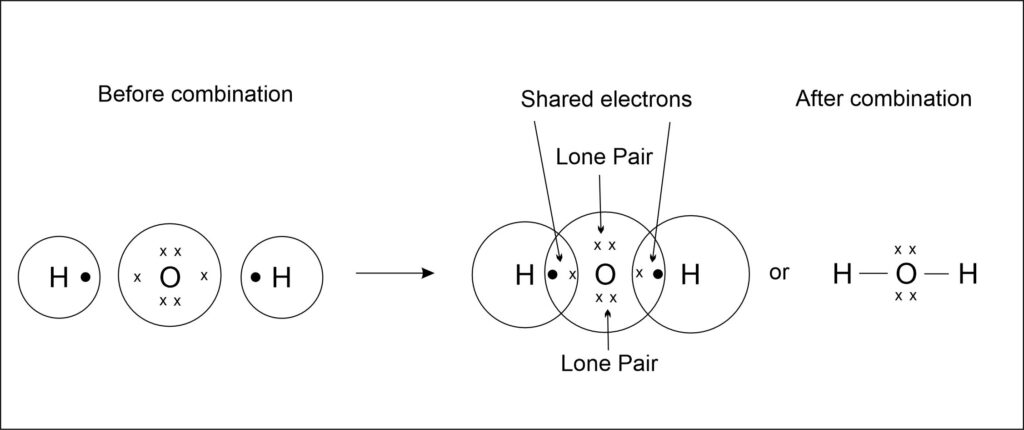
Note: Lone Pairs are the electron pairs of valence electrons which do not take part in chemical bonding. They only take part in the formation of coordinate bond between the atoms of two molecules.
Properties of Covalent Compounds
- Covalent compounds have low melting point and boiling points due to small intermolecular forces of attraction between the atoms.
- Covalent compounds are generally poor conductors of electricity. This is because the electrons are shared between atoms and no charged particles are formed in these compounds.
- Covalent compounds are generally volatile in nature.
- Covalent compounds show isomerism.
Allotropes of Carbon
Allotropy is the property by virtue of which an element exists in more than one form and each form has different physical properties but identical chemical properties. These different forms are called allotropes. Carbon exists in different allotropic forms: Diamond, graphite, fullerene etc.
Both diamond and graphite are formed by carbon atoms, the difference lies in the manner in which the carbon atoms are bonded to one another.
In diamond, each carbon atom is bonded to four other carbon atoms forming a rigid three-dimensional structure.
In graphite, each carbon atom is bonded to three other carbon atoms in the same plane giving a hexagonal array.
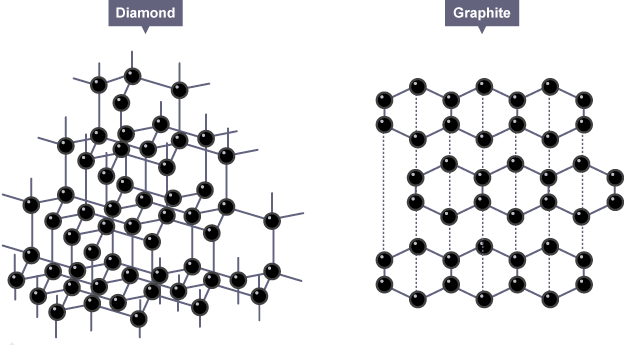
These different structures result in very different properties in diamond and graphite even though their chemical properties are the same.
Diamond is a colourless transparent substance and very hard whereas graphite is an opaque substance which have smooth surface and slippery to touch. Graphite is also a very good conductor of electricity unlike other non-metals.
Diamond can be synthesized by subjecting pure carbon to very high pressure and temperature. These synthetic diamonds are small but are otherwise indistinguishable from natural diamonds.
Fullerenes are recently discovered allotropic forms of carbon which were prepared for the first time by H. W. Kroto, Smalley and Robert Curt by the action of laser beam on the vapours of graphite.
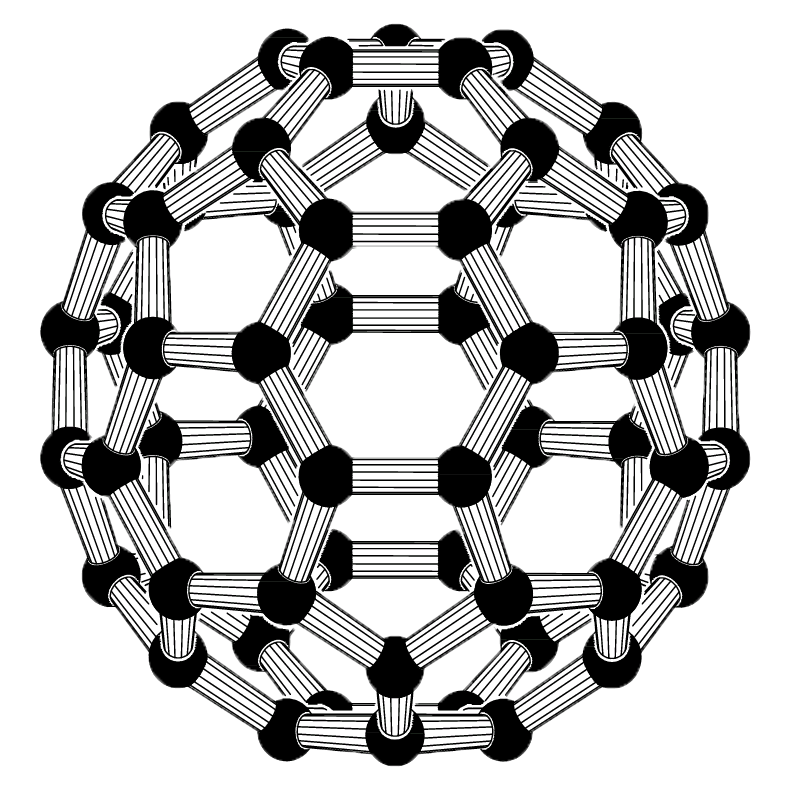
Versatile Nature of Carbon
Main factors that are responsible for the formation of large number of carbon compounds are
- Catenation: The property of self linking of elements mainly C – atoms through covalent bonds to form long, straight or branched chains and rings of different sizes is called catenation. Carbon shows maximum catenation in the periodic table due to its small size and strong C – C bond.
- Tetravalency of Carbon: The valency of carbon is four i.e., it is capable of bonding or pairing with four other carbon atoms or with the atoms of some other monovalent elements like hydrogen, halogen (chlorine, bromine) etc.
- Tendency to form multiple bonds: Carbon has a strong tendency to form multiple bonds due to its small size. It shares more than one electron pair with its own atoms or with the atoms of elements like oxygen, nitrogen, Sulphur etc.
Organic Compounds
Organic compound is any chemical compound that contain carbon–hydrogen or carbon-carbon bonds. In other words, the compounds of carbon except its oxides, carbonates and hydrogen carbonate salts, are known as organic compounds.
In 1828, German chemist Friedrich Wohler accidently prepared urea from ammonium cyanate when he was trying to prepare ammonium cyanate by heating ammonium sulphate and potassium cyanate. Thus, synthesis of urea discarded vital force theory.
Hydrocarbons
Organic compounds made up of carbon and hydrogen are called hydrocarbons. These are of two types; Aliphatic and Aromatic.
Types of Aliphatic Hydrocarbons
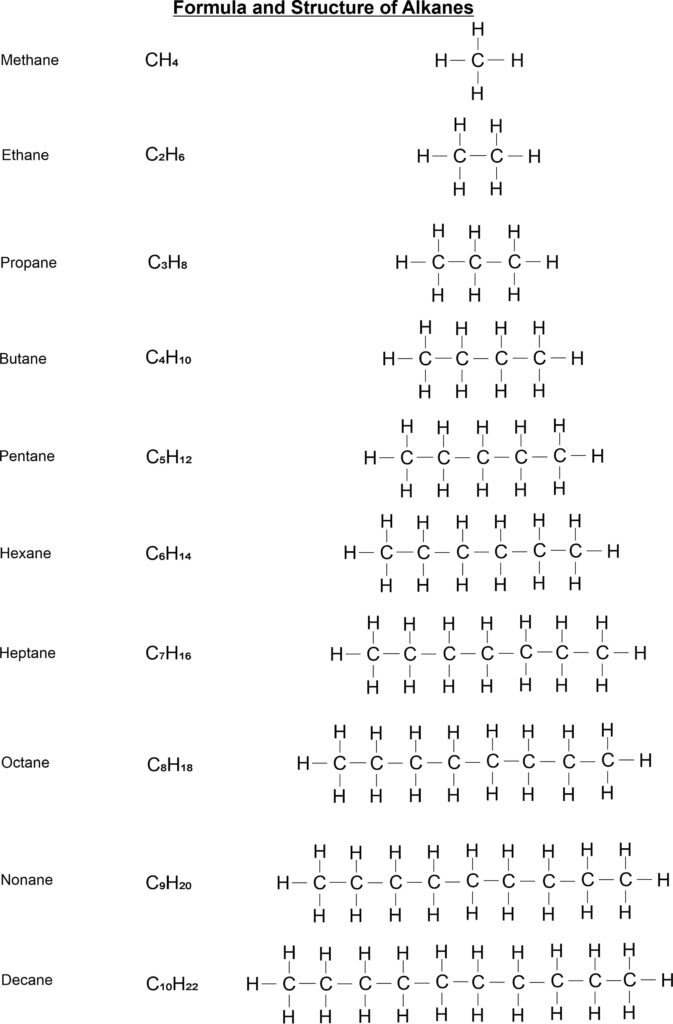
- Saturated Hydrocarbons: Compounds of carbon, which are linked by only single bonds between the carbon atoms are called saturated compounds. Alkanes or paraffin are called saturated hydrocarbons. The general formula of these compounds is CnH2n+2. Its IUPAC name is alkane. Examples, C2H6 (ethane), C3H8 (propane) etc.
- Unsaturated Hydrocarbons: Compounds of carbon having double or triple bonds between their carbon atoms are called unsaturated compounds. These are of two types:
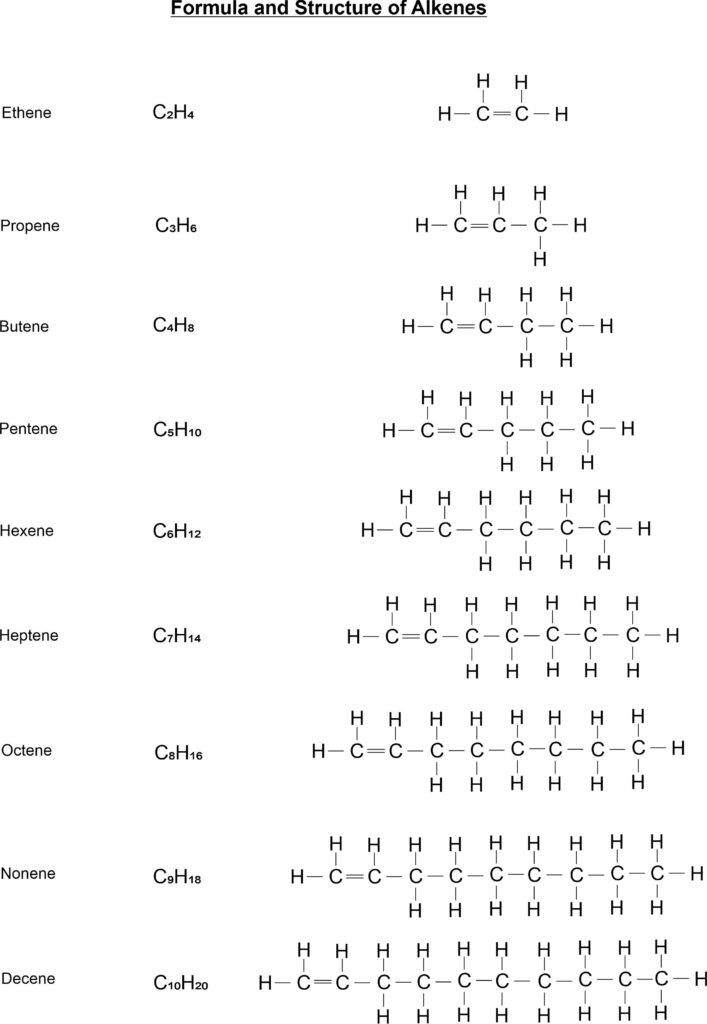
- Alkene: Those hydrocarbons in which at least one carbon – carbon double bond are called alkenes or olefins. The general formula of these compounds is CnH2n. Its IUPAC name is alkene. For examples, C2H4 (ethene), C3H6 (propene), C4H8 (butene) etc.
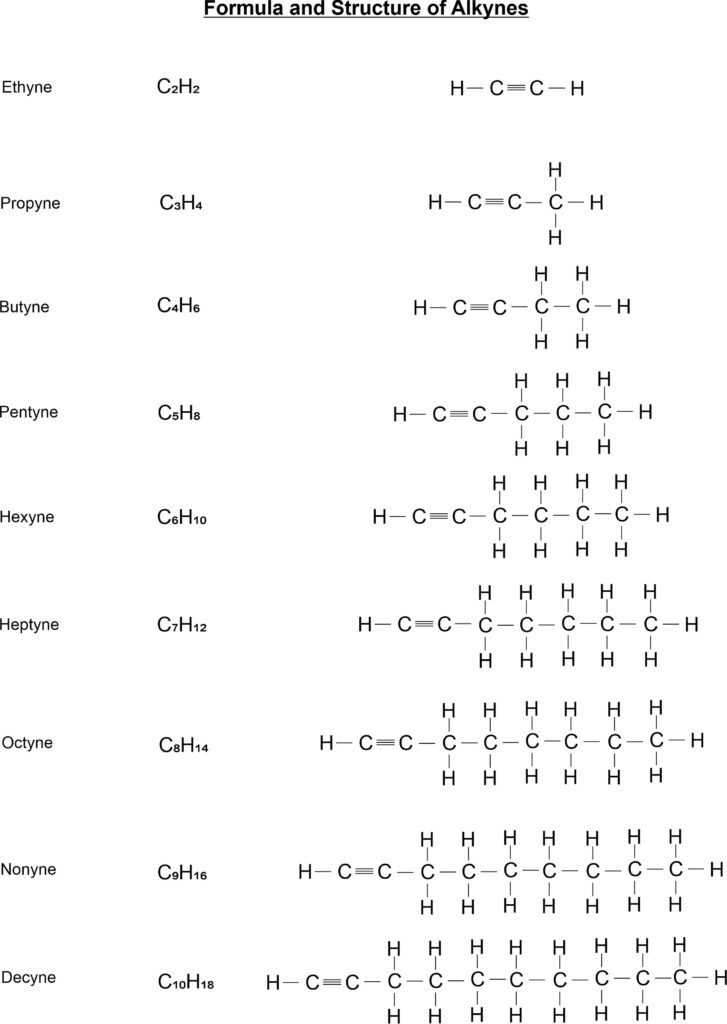
- Alkyne: Those hydrocarbons in which at least one carbon – carbon triple bond are called alkynes. The general formula of these compounds is CnH2n-2. Its IUPAC name is alkyne. For examples, C2H2 (ethyne), C3H4 (propyne), C4H6 (butyne) etc.

Straight chain structure of Alkanes
Straight chain structure of Alkenes
Straight Chain Structure of Alkynes
Structure of cycloalkane
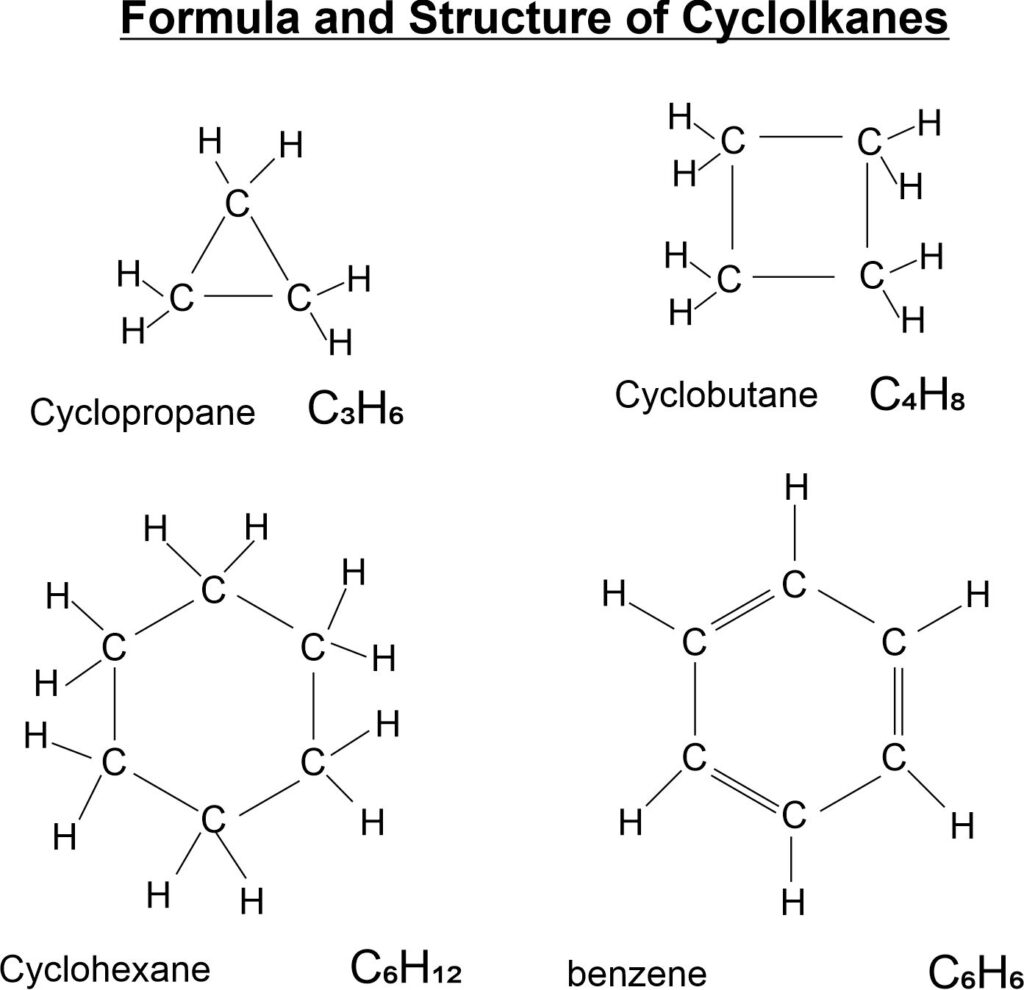
Note: Benzene is an aromatic hydrocarbon. Aromatic comes from aroma means sweet smell.
Functional Group
An atom or a group of atoms which gives some characteristics chemical properties to a compound is called a functional group.
Characteristics of functional group
- The functional group acts as the reactive site in the molecule.
- The chemical properties of the compounds containing the same functional group are similar.
- Physical and chemical properties of compounds containing different functional groups are different.
Different functional groups
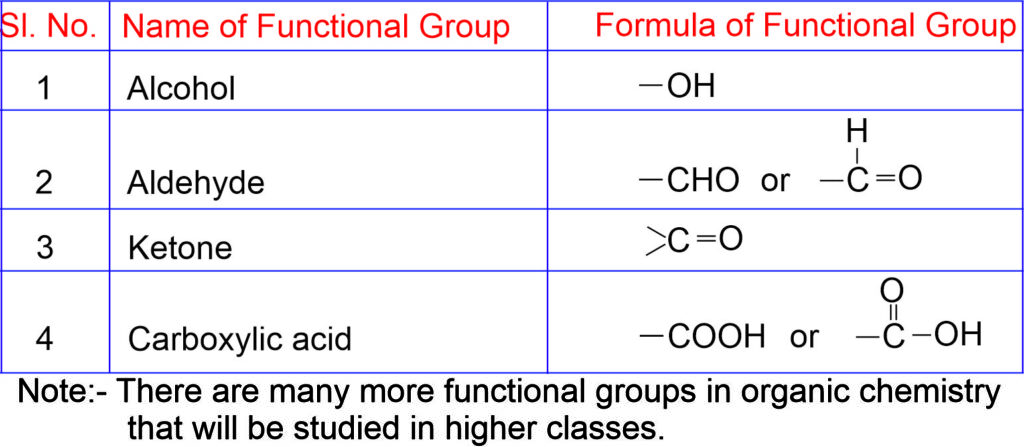
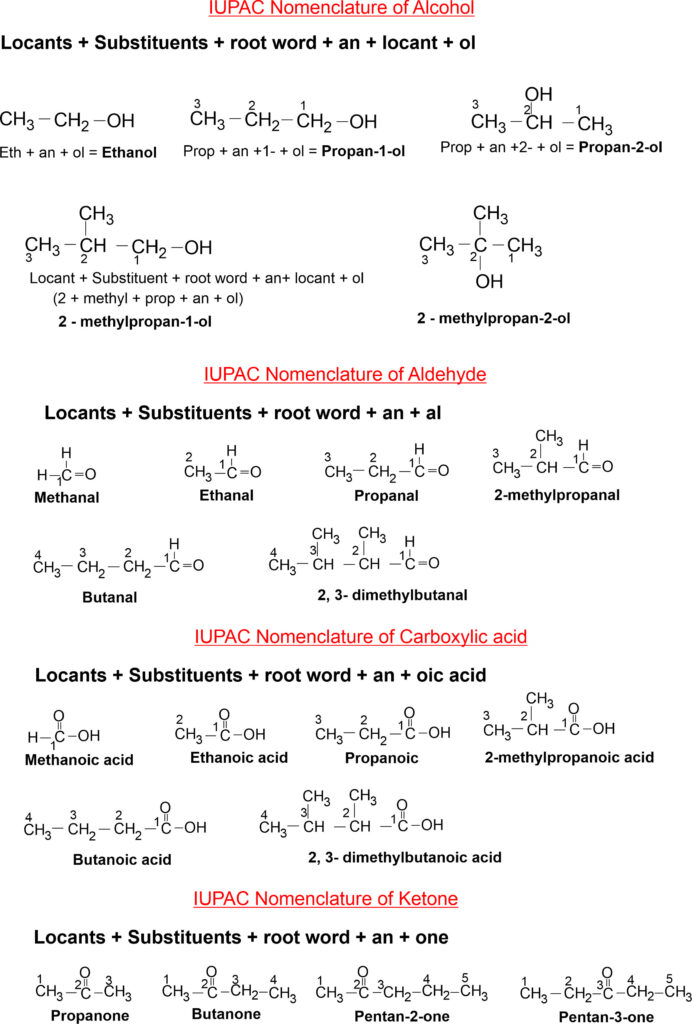
IUPAC Nomenclature of Carbon Compounds
Naming a carbon compound can be done by the following methods –
(i) Identify the number of carbon atoms in the atoms in the compound. Each number is given a specific name called root name (or word).
C1 – Meth, C2 – Eth, C3 – Prop, C4 – But, C5 – Pent, C6 – Hex, C7 – Hept, C8 – Oct, C9 – Non, C10– Dec,
C11 – Undec, C12 – Dodec, etc.
(ii) Depending upon the nature of hydrocarbon suffix is added after root words such as
(a) For Alkane – Root word + ane (suffix). For examples,
CH4 – Meth (for C1) + ane (as suffix) = Methane
C2H6 – Eth (for C2) + ane (as suffix) = Ethane
C3H8 – Prop (for C3) + ane (as suffix) = Propane, etc.
(b) For Alkene – Root word + ene (as suffix). For examples,
C2H4 – Eth (for C2) + ene (as suffix) = Ethene
C3H6 – Prop (for C3) + ene (as suffix) = Propene, etc.
(c) For Alkyne – Root word + yne (as suffix). For example,
C2H2 – Eth (for C2) + yne (as suffix) = Ethyne
C3H4 – Prop (for C3) + yne (as suffix) = Propyne, etc.
(iii) For naming a branched chain hydrocarbon the following rules are followed:
(a) The longest chain of carbon atoms in the structure of the molecule is selected. The longest chain is called parent chain and the hydrocarbon with parent chain is called parent hydrocarbon. The given compound is then named as a derivative of the parent hydrocarbon.
(b) The alkyl groups present in the side chain (as substituents) are numbered in such a way that the carbon atom having the substituent gets the lowest possible number.
(c) The IUPAC naming is given as:
Locant(s) + Substituents (in alphabetical order) + root word + suffix.
For examples,
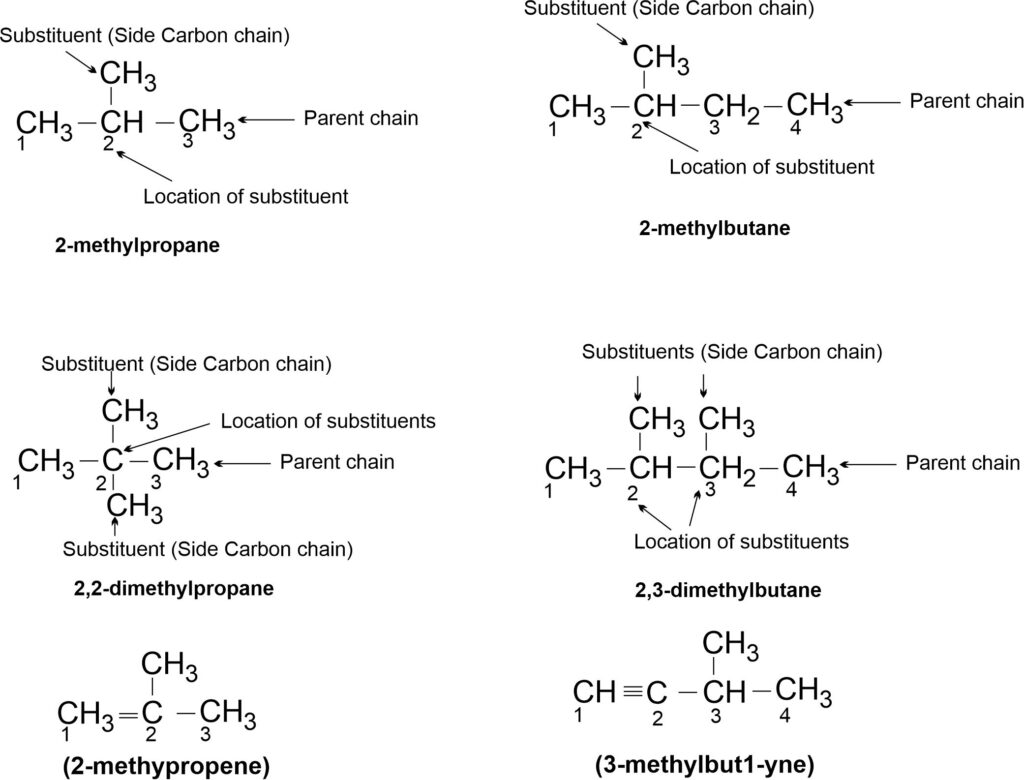
Note: In case of alkene or alkyne, numbering is done from the carbon having double or triple bond respectively.
(iv) For naming a compound having functional group, following rules are followed –
(a) In case of functional group having no carbon atom like – OH (alcohol), – X (halogens), the parent chain is chosen such that they acquire the lowest position.
(b) In case of functional group having carbon atom like – CHO, – COOH, etc., the numbering for parent chain starts from the carbon of functional groups.
For examples,
Homologous Series
A group of organic compounds containing a particular functional group is termed as a homologous series.
A member of any homologous series is called a homologue.
Characteristics of Homologous Series
(i) All the members of a homologous series can be described by a common general formula. For examples,
(a) Alkane is a homologous series having general formula: CnH2n+2. Similarly, alkene and alkyne has general formula CnH2n and CnH2n-2 respectively.
(b) Organic compound having functional group has general formula : CnH2n+1-(f.g). For examples – For alcohol, it is CnH2n+1 – OH; for aldehyde, it is CnH2n+1 – CHO; for carboxylic acid, it is CnH2n+1 – COOH and for ketone it is CnH2n+1 – CO – CnH2n+1.
(ii) Each member of a homologous series differs from its higher and lower neighbouring members by a common difference of – CH2.
(iii) All the properties of a homologous series show similar chemical properties.
(iv) Physical properties in a homologous series show a regular variation with an increase in molecular mass. For examples,
(a) With increase in molecular mass, the melting point and boiling points increase.
(b) For Alkane, lower members (C1-C4) have gaseous states, intermediate (C5 – C17) have liquid states while the further higher members (C18 – onwards) have solid states.
Isomerism
Organic compounds with same molecular formula but different properties due to different arrangement of atoms in the molecule are called isomers. This phenomenon is called isomerism.
Characteristics of Isomerism:
- Isomers have same molecular formula.
- Isomers have different structural formulae.
- Isomers have different physical and chemical properties.
- Isomers show different properties due to the different arrangement of carbon atoms in their molecules.
Different types of Isomerism:
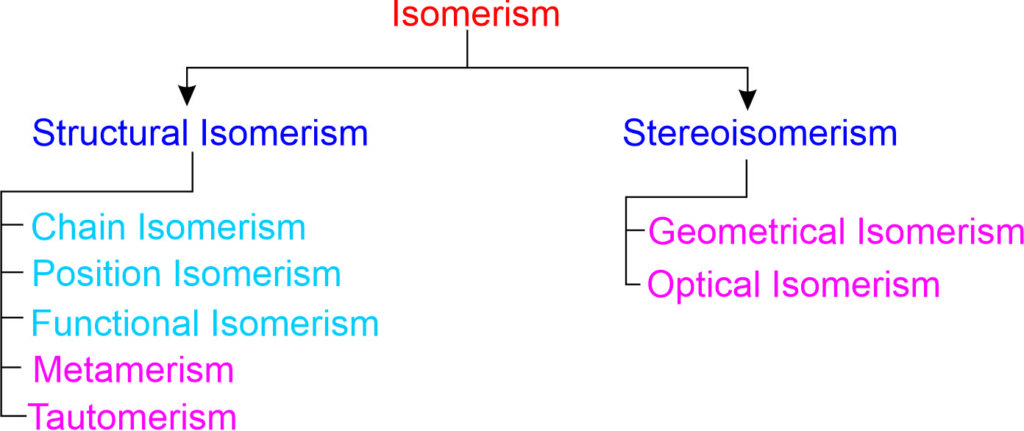
Structural Isomerism
The structural isomerism arises due to the difference in the arrangement of atoms within the molecules i.e., due to different structure of same molecule.
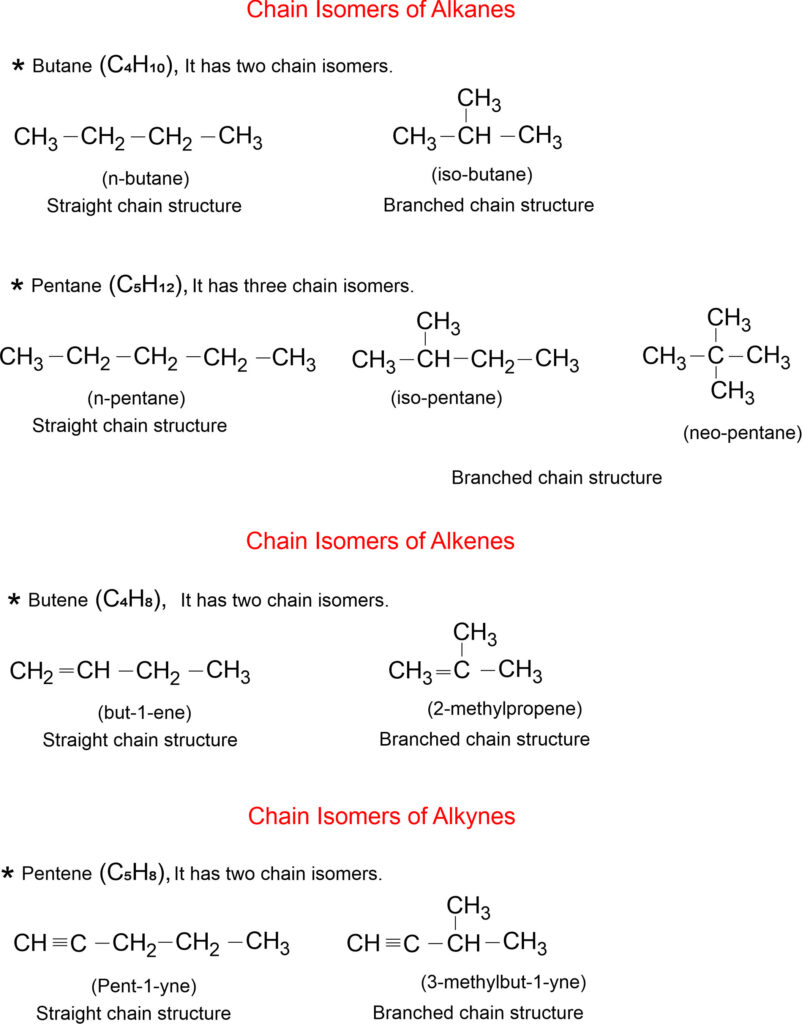
Chain Isomerism
Chain isomerism occurs when there is a difference in the atomic arrangement of the carbon to the carbon chain of a molecule. If two or more compounds having the same type of molecular formula with different main chains, then they are said to exhibit the property of Chain isomerism.
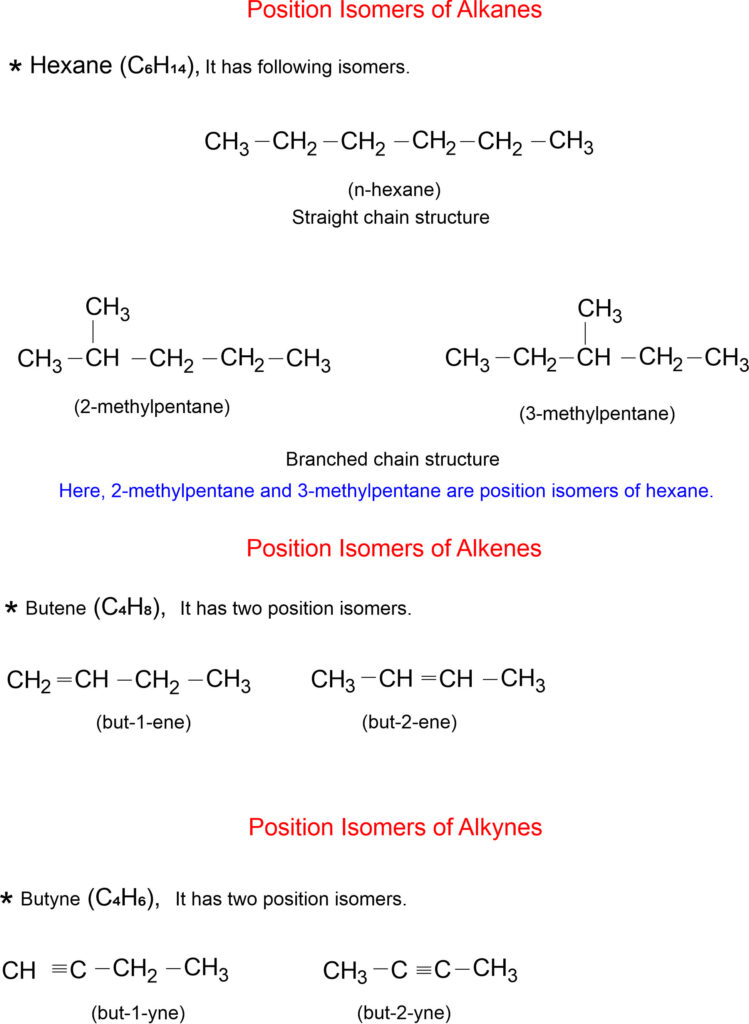
Position Isomerism
This isomerism arises due to difference in the position of the same functional group or the same substituent on the chain. The arrangement of carbon atoms remains the same.
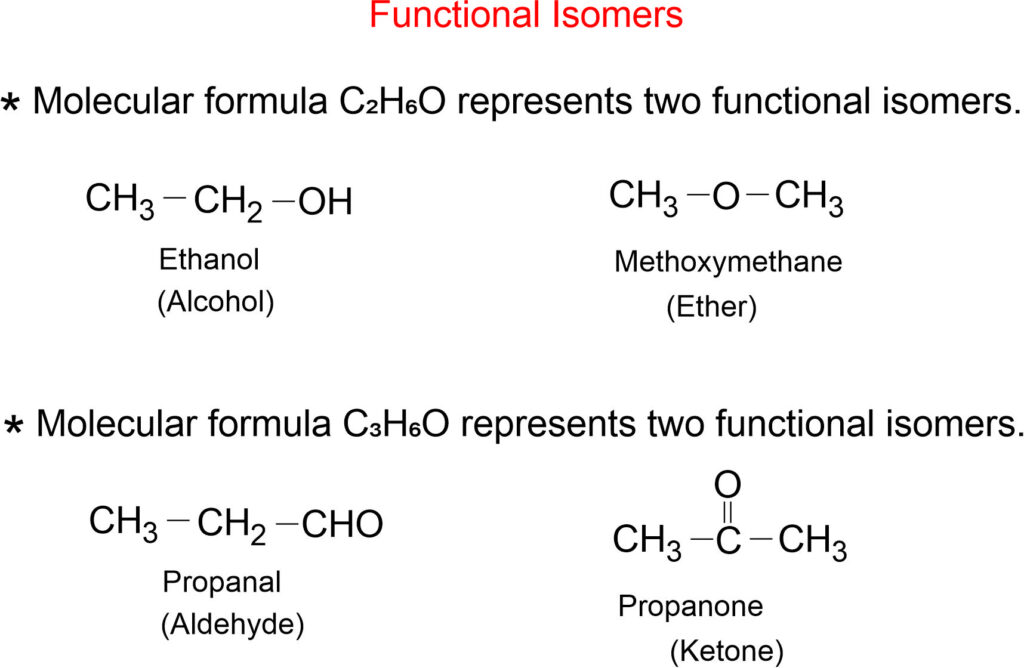
Functional Isomerism
The compounds having the same molecular formula but different functional groups are said to exhibit functional isomerism. Such compounds are termed as functional isomers.
Chemical Properties of Carbon and Its Compounds
Combustion
A chemical reaction in which a substance is burnt in the presence of oxygen and produces heat and light (in the form of flame) is called combustion.
In other words, it is a rapid oxidation/burning of any substance in which heat and light are produced.
For example,
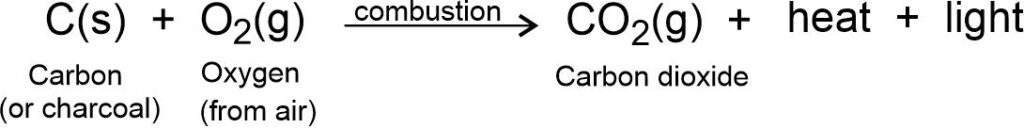
Combustion of carbon: Carbon (or charcoal, coal, coke etc.) burns in air or oxygen to give CO2 (Carbon dioxide gas), heat and light.
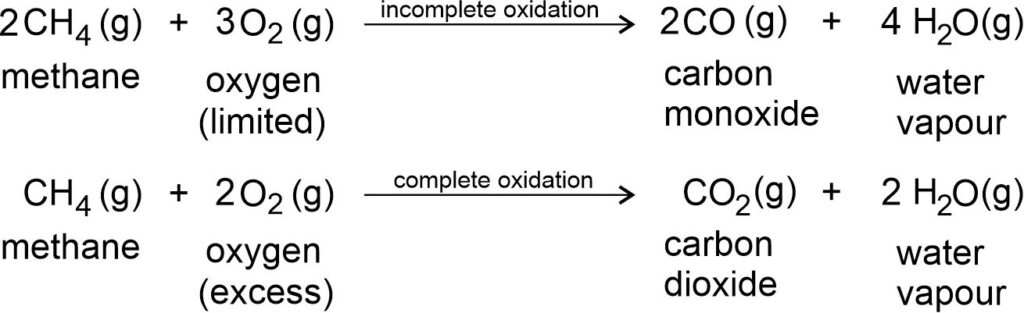
Combustion of hydrocarbon: Hydrocarbon burns to produce carbon dioxide (CO2), water (H2O), heat and light.
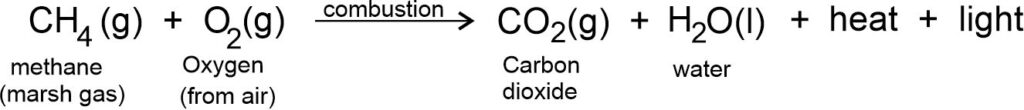
Natural gas and biogas contain methane. So, burning of these gases are also combustion reactions.

Similarly, burning of kerosene, petrol, diesel, crude oil etc. produce carbon dioxide (CO2), water (H2O), heat and light.
In general, the combustion of hydrocarbons has the balanced chemical equation as:
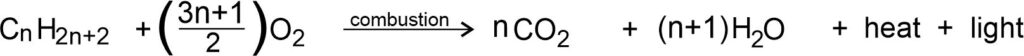
Burning of alcohol: Alcohol also, burns to give carbon dioxide (CO2), water (H2O), heat and light.
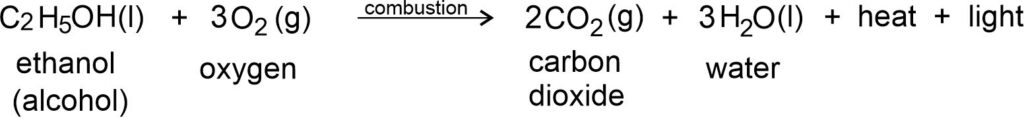
Nature of Flame:
- Saturated hydrocarbons such as methane, ethane, propane, butane and natural gas and LPG burn with a blue flame due to their complete combustion in the presence of sufficient oxygen (air) but in the limited amount of oxygen (air), they burn with sooty and smokey flame due to incomplete combustion. For example, gas stove using LPG gives blue flame and kerosene when burnt in a pressure stove gives blue flame.
- Unsaturated hydrocarbons such as ethene, propene, ethyne, propyne etc., give a yellow flame with lots of black smoke.
- Certain aromatic hydrocarbons like benzene, naphthalene etc., burn with a sooty flame, giving thick black clouds of smoke.
Oxidation
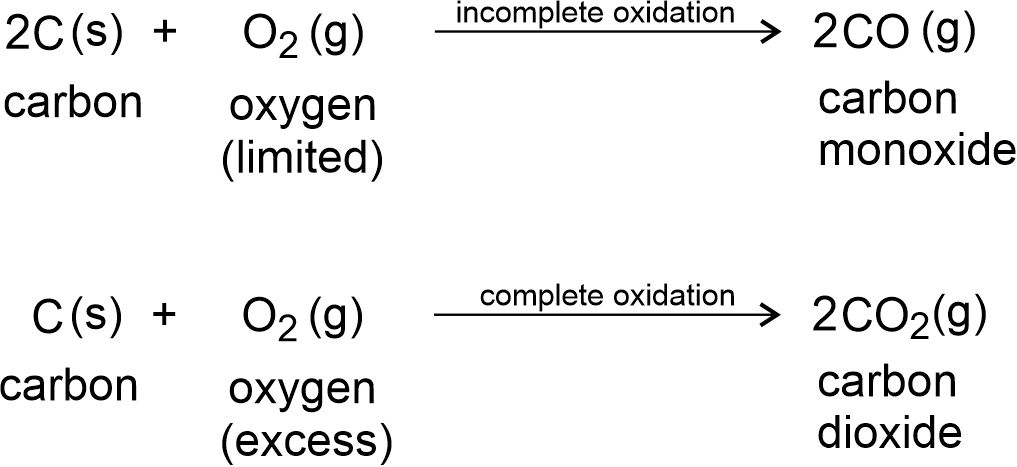
The reaction involving the addition of oxygen and removal of hydrogen is called oxidation reaction. Carbon and its compounds can be easily oxidised on combustion (or burning). During combustion, the compound gets oxidised completely to the product of highest oxidation level, i.e., carbon dioxide. For example,
Oxidation of Carbon: Carbon gives carbon monoxide (CO) with limited oxygen and carbon dioxide (CO2) with excess oxygen.
Oxidation of hydrocarbons: They also give different products in different conditions.
Oxidation of Alcohols: Alcohol gives different products on oxidation depending upon the reaction conditions.
When alcohols are heated with alkaline KMnO4 (potassium permanganate) or acidified K2Cr2O7 (potassium dichromate), alcohols give carboxylic acids containing same number of carbon atoms.
For examples,
Ethanol is converted into ethanoic acid on oxidation in the presence of alkaline KMnO4 (potassium permanganate) or acidified K2Cr2O7 (potassium dichromate).
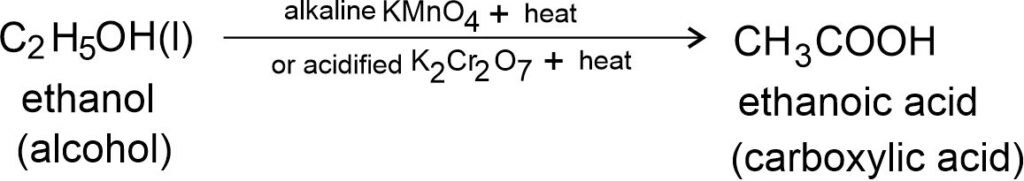
Here, alkaline potassium permanganate or acidified potassium dichromate oxidising alcohols to acids i.e., adding oxygen to the alcohols. Hence, they are known as oxidising agents.
Addition Reactions
The reaction in which a reagent completely add to a reactant without the removal of small molecules are called addition reaction.
All unsaturated hydrocarbons contain one or more double or triple bonds. These double or triple bonds have tendency to get converted into single bond by adding small molecules, such as hydrogen (H2), halogen (X2 i.e., Cl2, Br2, and I2), water (H2O) and halogen acids (HX i.e., HCl, HBr, HI ) across them. These reactions are called addition reactions.
For example,
Hydrogenation: Addition of hydrogen in the presence of catalysts like palladium (Pd) or Nickel (Ni), to unsaturated hydrocarbons (alkene or alkyne) yields saturated hydrocarbons (alkane and its derivatives).
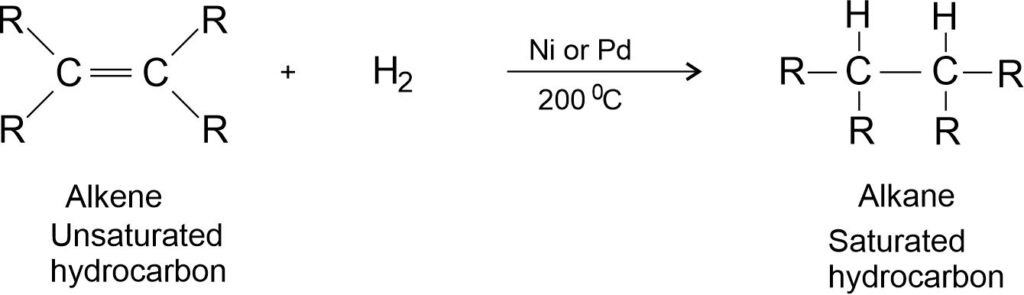
Note: Catalysts are substances that cause a reaction to occur or proceed at a different rate without the reaction itself being affected.
Here, R is the alkyl groups like methyl (CH3), ethyl (C2H5) etc. Here, R can also be replaced by H. For example,
Addition of hydrogen to ethene:
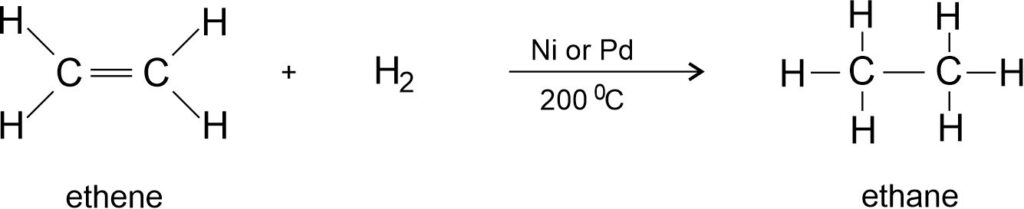
Addition of hydrogen to ethyne:
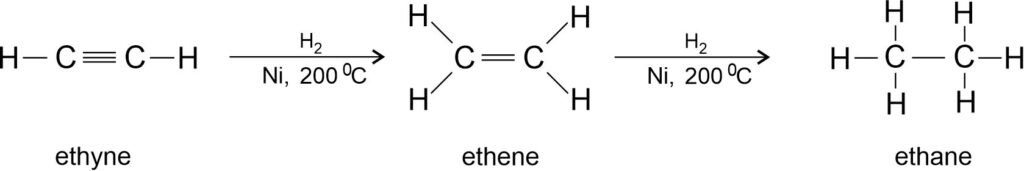
Hydrogenation reaction is commonly used to convert vegetable oil (unsaturated) such as groundnut oil, cotton seed oil to vegetable ghee (saturated). The process of converting a vegetable oil into a solid fat (vegetable ghee) is called hydrogenation of oils.
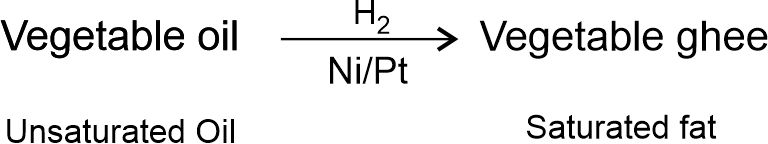
Note: Animal fats generally contain saturated fatty acids which are said to be harmful for health. Oil containing unsaturated fatty acids should be chosen for cooking.
Substitution Reaction
The reactions in which one or more hydrogen atoms of a molecule are replaced by some other atoms or groups are called substitution reactions.
Saturated hydrocarbons are fairly unreactive and are inert in the presence of most reagents. However, in the presence of sunlight, chlorine is added to hydrocarbons in a very fast reaction. Reaction continues till all the hydrogens get replaced by equal number of chlorine atoms.
The substitution reaction in which one or more hydrogen atoms are replaced by an equal number of halogen atoms is called halogenation.
For example,
Chlorination of methane:
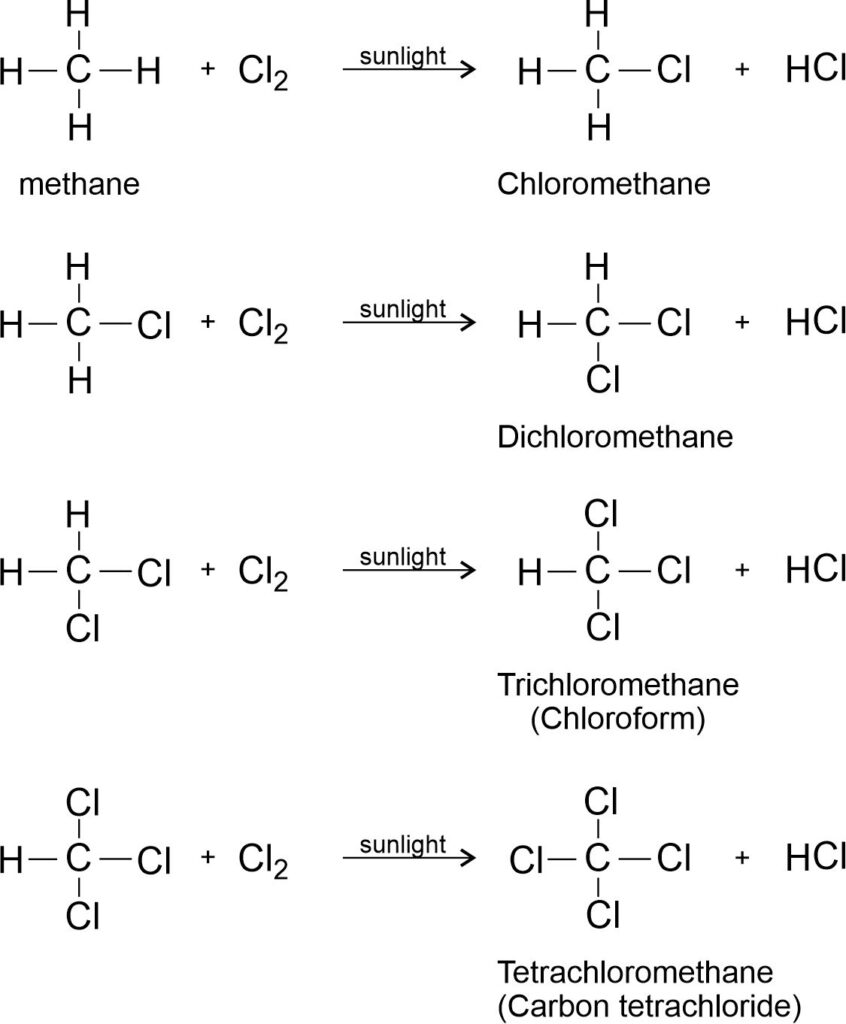
Note: Chloroform (CHCl3) is a colorless liquid that quickly evaporates into gas. It can harm the eyes, skin, liver, kidneys, and nervous system. Chloroform can be toxic if inhaled or swallowed.
Carbon tetra chloride (CCl4) is an organic solvent used to dissolve non-polar organic solutes.
Properties of Ethanol (Ethyl alcohol), C2H5OH
Physical properties:
- Its melting point and boiling point are 156 K and 351 K respectively.
- Ethanol is a colourful, inflammable liquid with a spirituous odour and burning taste.
- It is commonly called alcohol and is the active ingredient of all alcoholic drinks.
- It is a good solvent so it is used in medicines such as tincture iodine, cough syrups, and many tonics.
- It is also soluble in water in all proportions.
Note: Consumption of small quantities of dilute ethanol causes drunkenness. Intake of even a small quantity of pure ethanol (called absolute alcohol) can be lethal. When large quantities of ethanol are consumed, it tends to slow metabolic processes and to depress the central nervous system. This results in the lack of coordination, mental confusion, drowsiness, lowering of the normal inhibitions, and finally stupour. The individual may feel relaxed but does not realise that his sense of judgement, sense of timing, and muscular coordination have been seriously impaired.
Chemical properties:
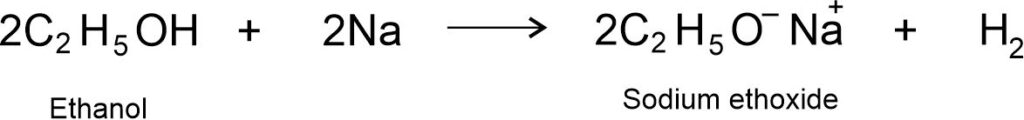
(i) Reaction with Metals: Ethanol reacts with highly reactive metals like Na, K, etc. to produce hydrogen gas. The second product is metal ethoxide. For example, ethanol reacts with sodium to produce sodium ethoxide and hydrogen gas.
(ii) Dehydration of ethanol: Heating of heated at 443 K with excess concentrated sulphuric acid results dehydration of ethanol to give ethene.
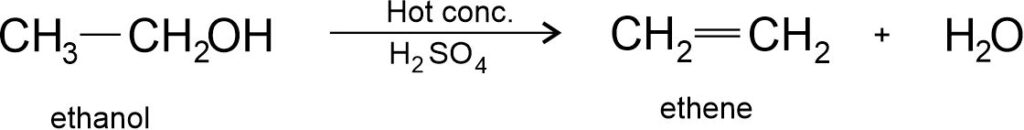
The conc. sulphuric acid can be regarded as a dehydrating agent which removes water from ethanol.
Properties of Methanol
- It is a flammable, light, poisonous liquid.
- It has a distinctive odour which is milder and sweeter than ethanol. It is volatile and does not have colour.
- Intake of methanol in very small quantities can cause death.
- Methanol is oxidised in the liver. It reacts rapidly with the components of cells. It causes the protoplasm to get coagulated, in much the same way as the egg is coagulated by cooking. It also affects the optic nerve, casing blindness.
Note: To prevent the misuse of ethanol produced for industrial use, it is made unfit for drinking by adding poisonous substances like methanol into it. Dyes are also added to colour the alcohol blue so that it can be identified easily. This is called denatured alcohol.
Alcohol as a Fuel
Sugarcane plants are one of the most efficient converters of sunlight into chemical energy. Sugarcane juice can be used to prepare molasses which is fermented to give alcohol (ethanol).
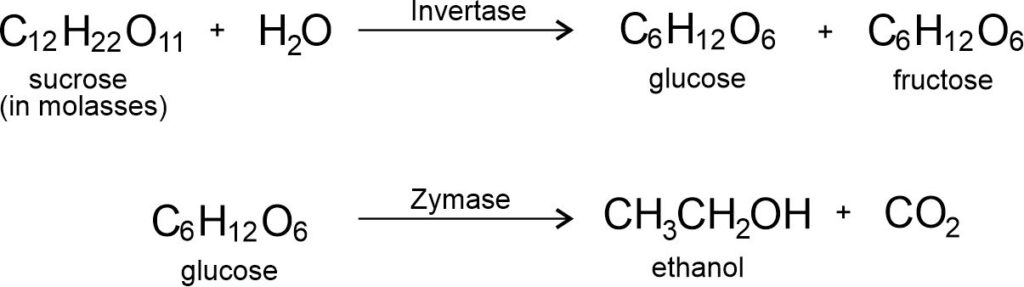
This alcohol is used as an additive in petrol. It is a cleaner fuel which gives rise to only carbon dioxide and water on burning in sufficient air (oxygen).
Uses of Ethanol
(i) As a fuel for lamps and stoves.
(ii) As a solvent for drugs, tinctures, oils, perfumes, inks, dyes, varnishes, etc.
(iii) As an antifreeze for automobile radiators.
(iv) As a preservative for biological specimens.
(v) As an antiseptic to sterilize wounds and syringes in hospitals.
Ethanoic acid
Its common name is acetic acid and is second member of homologous series called carboxylic acid. Its chemical formula is CH3COOH.
Ethanoic acid is well known for over centuries in the form of vinegar. Vinegar contains about 5-8% of ethanoic acid. Vinegar is widely used as a preservative in pickles.
In the combined state as salt or ester, it is present in biological fluids and plant extracts.
Physical Properties of ethanoic acid
(i) At ordinary temperature, ethanoic acid is a colourless liquid with a strong pungent smell and sour taste.
(ii) On cooling below 16.5 0C, it forms ice-like crystals. So, it is also called as glacial acetic acid.
(iii) It is weaker acid than HCl but stronger than alcohol.
(iv) It has a corrosive action on the skin and cause blisters.
(v) It is miscible in water due to formation of hydrogen bonds with water molecules.
Chemical properties of ethanoic acid
(i) Esterification reaction: Ethanoic acid reacts with absolute ethanol in the presence of an acid catalyst to give an ester.
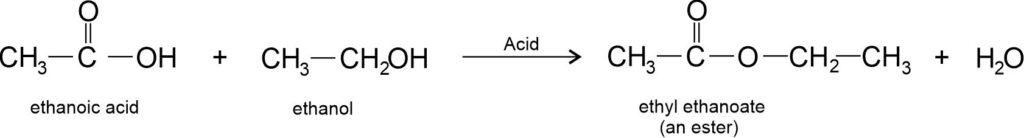
Note: Esters are sweet-smelling substances. These are used in making perfumes and as flavouring agents. Esters react in the presence of an acid or a base to give back the alcohol and carboxylic acid. This reaction is known as saponification because it is used in the preparation of soap.
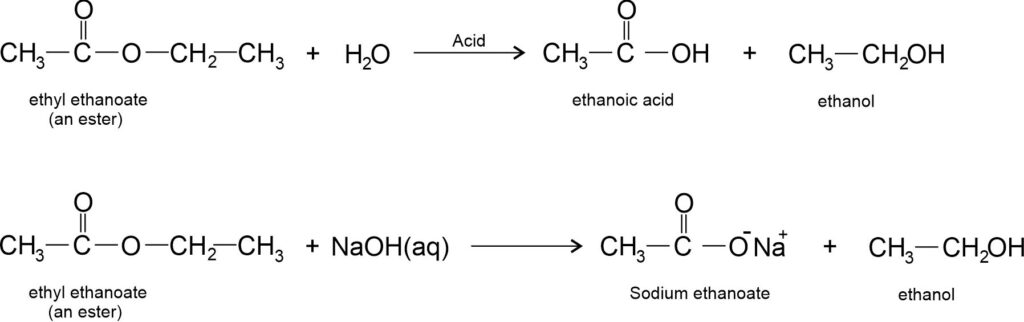
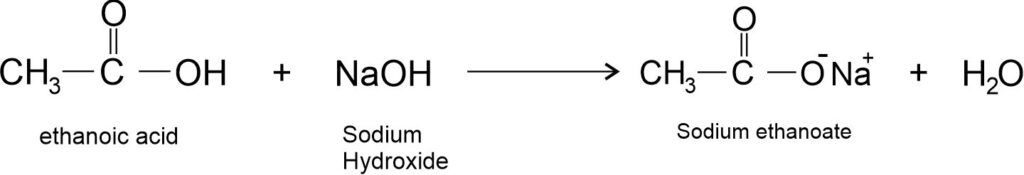
(ii) Reaction with base: Ethanoic acid reacts with a base (like NaOH) to give a salt (sodium ethanoate or commonly called sodium acetate) and water.
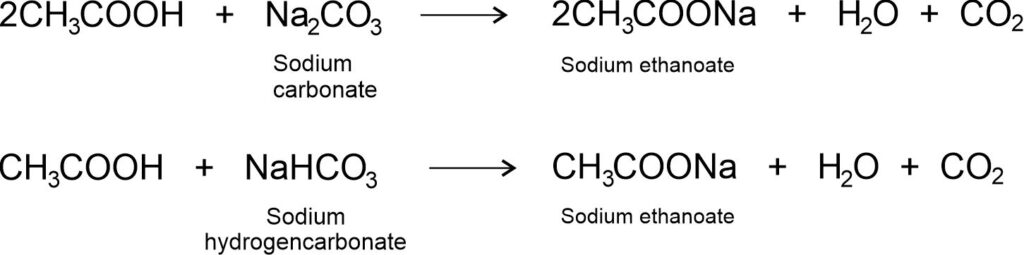
(iii) Reaction with carbonates and hydrogencarbonates: Ethanoic acid reacts with carbonates and hydrogencarbonates to give rise to a salt, carbon dioxide and water.
Uses of ethanoic acid
(i) In the manufacture of various dyes, perfumes and rayon.
(ii) Salts of ethanoic acids are used in paints and also in certain medicines.
(iii) For making synthetic vinegar which is used in pickles, etc.
(iv) As a solvent.
Soaps and Detergents
Soaps are sodium and potassium salts of long chain carboxylic acids. Soaps have general formula RCOO–Na+, R is an alkyl group containing 15 to 18 carbon atoms. For example, C15H31, C17H35, etc.
Detergents are usually ammonium or sulphonate salts of long chain carboxylic acids. They are also called nonsoapy or soapless soap.
Note: Common soap is a typical detergent. When we talk of a detergent, we really mean a synthetic detergent.
Synthetic detergents
Sodium salts of sulphonic esters are called synthetic detergents. For example,
(i) Linear alkyl benzenesulphonate: R-C6H4-SO3–Na+, where R is a long chain alkyl group. The most common detergent in this class is sodium n-dodecylbenzenesulphonate i.e., C12H25-C6H4-SO3–Na+.
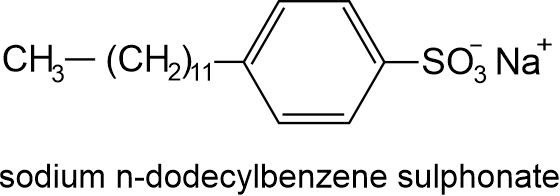
(ii) Sodium lauryl sulphate i.e., C12H25OSO3–Na+
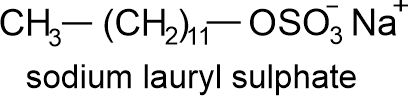
Preparation of soap and detergent
Soaps are made from animal fats or vegetable oils by heating it with sodium hydroxide. This process is called saponification.
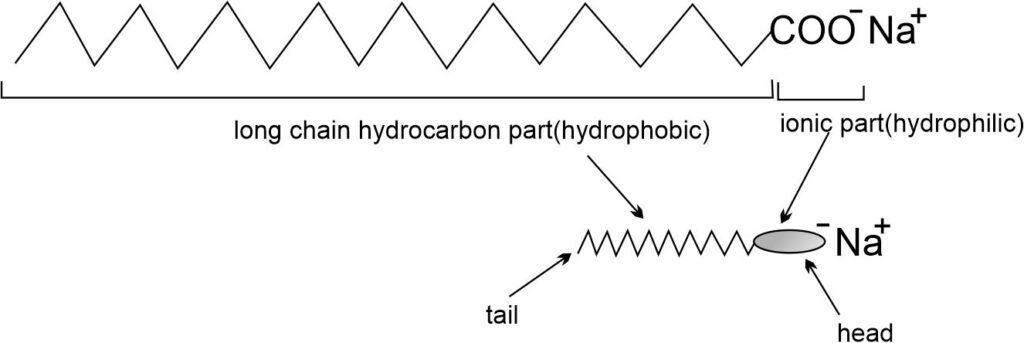
Structure of Soap Molecule
A molecule of soap is made up of two parts – a long chain hydrocarbon part (or non-ionic part or hydrocarbon end) and a short ionic part (or polar end) containing -COO–Na+ group.
The polar end of soap -COO–Na+ is water-soluble i.e., hydrophilic but insoluble in oil, whereas the hydrocarbon end is water-repellent i.e., hydrophobic but soluble in oil.
Cleansing action of Soap
When an oily (dirty) piece of cloth is put into soap solution, the hydrocarbon part of the soap molecule attaches itself to the oily drop, and the ionic part -COO– orients itself towards water. The Na+ ions in solution arrange themselves around the -COO– ions. The negatively charged micelle so formed entraps the oily dirt. The negatively charged micelles repel each other due to the electrostatic repulsion. As a result, the tiny oily dirt particles do not come together and end get washed away in water during rinsing.
Mechanism of Micelle Formation
Soaps are molecules in which the two ends have different properties, one is hydrophilic, that is, it dissolves in water, while the other end is hydrophobic, that is, it dissolves in hydrocarbons.
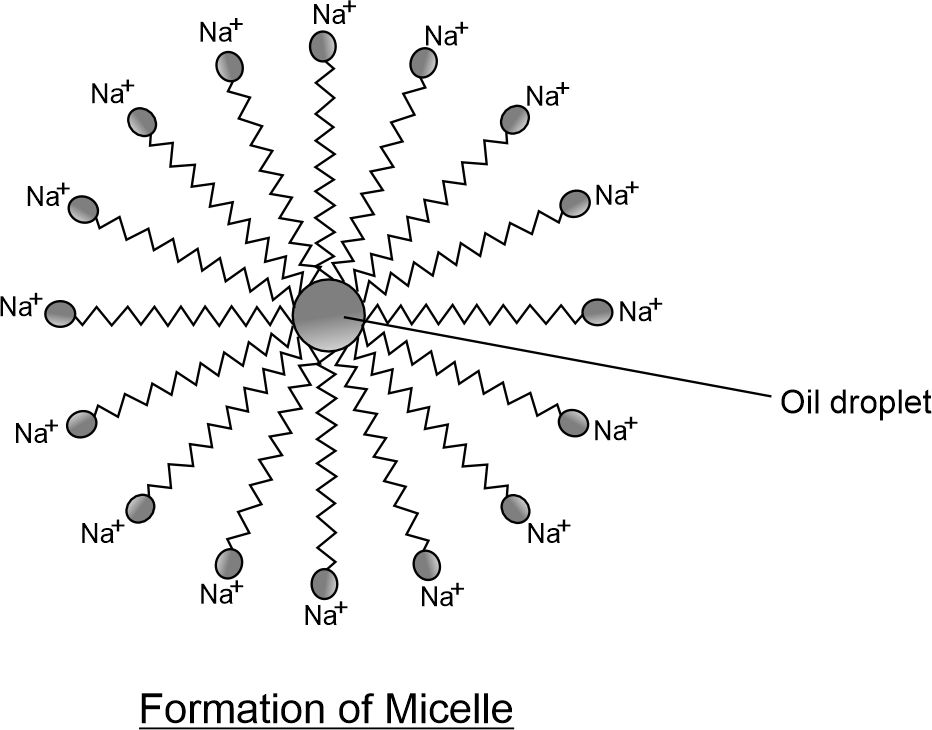
When soap is at the surface of water, the hydrophobic ‘tail’ of soap will not be soluble in water and the soap will align along the surface of water with the ionic end in water and the hydrocarbon ‘tail’ protruding out of water. Inside water, these molecules have a unique orientation that keeps the hydrocarbon portion out of the water. This is achieved by forming clusters of molecules in which the hydrophobic tails are in the interior of the cluster and the ionic ends are on the surface of the cluster. This formation is called a micelle.
Soap in the form of a micelle is able to clean, since the oily dirt will be collected in the center of the micelle. The micelles stay in solution as a colloid and will not come together to due to electrostatic repulsion. Thus, the dirt suspended in the micelles is also easily rinsed away. The soap micelles are large enough to scatter light. Hence a soap solution appears cloudy.
Advantage of Detergents over Soaps
Sometime while bathing the foam is formed with difficulty and an insoluble substance (scum) remains after washing with water because of the reaction of soap with the calcium and magnesium salts, which cause the hardness of water. Hence, a large amount of soap get wasted. This problem is overcome by using detergents instead of soaps as cleansing agents. Detergents are generally sodium salts of sulphonic acids or ammonium salts with chlorides or bromides ions etc. Both have long hydrocarbon chain. The charged ends of these compounds do not form insoluble precipitates with the calcium and magnesium ions in hard water. Thus, they remain used to make shampoos, and products for cleaning cloths.
Questions
Q.1. A mixture of oxygen and ethyne is burnt for welding. Can you tell why a mixture of ethyne and air is not used?
Ans: Ethyne (C2H2) has higher percentage of carbon. Oxygen present in the air is about 21%. This quantity of oxygen is not sufficient for the complete combustion of ethyne. For welding, a higher temperature is needed. So, to ensure the complete combustion of ethyne, oxygen is used in the welding torch.
Q.2. How would you distinguish experimentally between an alcohol and a carboxylic acid?
Ans: Alcohol and carboxylic acid can be distinguish experimentally by two reactions:
(i) Reaction with Base: Alcohols do not react with base like NaOH, KOH while carboxylic acids react with base to form salt and water. For example,
CH3COOH + NaOH → CH3COO–Na+ + H2O
CH3CH2OH + NaOH → No reaction
(ii) Reaction with Sodium bicarbonate: Carboxylic acids react with sodium bicarbonate rapidly togive brisk effervescence of CO2 gas while alcohols do not react. For example,
CH3COOH + NaHCO3 → CH3COONa + H2O + CO2
CH3CH2OH + NaHCO3 → No reaction.
Q.3. Would you able to check if water is hard by using a detergent?
Ans: No, because detergents give lather even in hard water.
Q.4. People use a variety of methods to wash clothes. Usually after adding the soap, they ‘beat’ the clothes on a stone, or beat them with a paddle, scrub with a brush or the mixture is agitated in a washing machine. Why is agitation necessary to get clean clothes?
Ans: Agitation helps in the removal of the dirt particles from the surface of the fabric. When soap solution is made and dirty clothes are soaked in it then micelles formation occurs. This results in the formation of an emulsion (a stable suspension of small droplets of one liquid in another with which it is immiscible). To wash away the loosen dirt particles in the form of micelles from the surface of the cloth, it is either scrubbed mechanically or beaten of agitated in washing machine.
Q.5. What change will you observe if you test soap with litmus paper (red or blue)?
Ans: Soap is alkaline. Hence, it turns red litmus blue and has no effect on the blue litmus paper.
Q.6. Why does micelle formation take place when soap is added to water? Will a micelle be formed in other solvents such as ethanol also?
Ans: When soap is at the surface of water, the hydrochloric ‘tail’ of soap will not be soluble in water and the soap will align along the surface of water with the ionic end in water and the hydrocarbon ‘tail’ protruding out of water. Inside water, these molecules have a unique orientation that keeps the hydrocarbon portion out of the water. This is achieved by forming clusters of molecules in which the hydrophobic tails are in the interior of the cluster and the ionic ends are on the surface of the cluster. This formation is called micelle.
Micelle will not formed in all types of solvents. It will form in such type of solvent in which soap is soluble. For example, soap in insoluble in ethanol, hence, no micelle formation take place.
Q.7. Explain the formation of scum when hard water is treated with soap.
Ans: Hard water contains calcium and magnesium ions. Soap is basically sodium or potassium salt of higher fatty acids. When soap is added to hard water, corresponding calcium and magnesium slats are formed, which being insoluble gets precipitated. These precipitates are called scum.
Reactions involved are:
$$\mathrm{\underset{from\ hard\ water}{Ca^{2+}}} \ +\mathrm{\underset{Soap}{2RCOONa}} \ \ \longrightarrow \mathrm{\underset{Calcium\ salt\ precipitate}{\ \ ( RCOO)_{2} Ca}} \downarrow \ +\ \ \mathrm{\underset{Sodium\ ion}{2Na^{+}}}$$
$$\mathrm{\underset{from\ hard\ water}{Mg^{2+}}} \ +\mathrm{\underset{Soap}{2RCOONa}} \ \ \longrightarrow \mathrm{\underset{Magnesium\ salt\ precipitate}{\ \ ( RCOO)_{2} Mg}} \downarrow \ +\ \ \mathrm{\underset{Sodium\ ion}{2Na^{+}}}$$
Q.8. Give a test that can be used to differentiate between butter and cooking oil.
Ans: Butter contains saturated compounds like cooking oil contains unsaturated compounds. Since, unsaturated compounds are oxidised by alkaline KMnO4 with disappearance of its pink colour. Therefore, when cooking oil is treated with a few drops of alkaline KMnO4, pink colour of KMnO4 disappears. With butter, however, the pink colour of KMnO4 does not disappear.
Previous Year Questions
Very Short Questions
Q.1. How is scum formed? [2012]
Q.2. Write the name and formula of the second member of the carbon compounds having functional group -OH. [2012]
Q.3. State the part of soap molecule that attaches itself to dirt when soap is dissolved in water. [2013]
Q.4. Write molecular formula of alcohol which can be derived from butane. [2015]
Q.5. Write the name of structure of an alcohol with three carbon atoms in its molecule. [2016]
Q.6. Select saturated hydrocarbons from the following: [2016]
C3H6; C5H10; C4H10; C2H4; C6H14
Q.7. How are covalent bonds formed? [2020]
Short Questions
Q.1. An organic acid X is a liquid which often freezes during winter time in cold countries. It has molecular formula C2H4O2. On warming with ethanol in the presence of a few drops of conc. H2SO4, a compound Y with sweet smell is formed.
(i) Identify X and Y.
(ii) Write the chemical equation for the reaction involved. [2011]
Q.2. (i) The formula of an ester is CH3COOC2H5. Write the structural formulae of the corresponding alcohol and the acid.
(ii) (a) Mention the experimental conditions involved in obtaining ethene form ethanol.
(b) Write the chemical equation for the above reaction. [2011]
Q.3. A compound X is used in cough syrups and many tonics. It is also soluble in water in all proportions:
(i) Name the compound X. Write its chemical formula.
(ii) Which gas is evolved when the compound X reacts with sodium? How will you test the presence of this gas? Write the chemical equation involved in reaction of X with sodium.
(iii) complete the following equation for X and identify Y.[2012]
$$X\ \mathrm{\xrightarrow[Heat]{Alk.\ KMnO_{4}}} \ Y$$
Q.4. A compound X is formed by the reaction of a carboxylic acid C2H4O2 and an alcohol in the presence of a few drops of H2SO4. The alcohol on oxidation with alkaline KMnO4 followed by acidification gives the same carboxylic acid as used in this reaction. Give the names and structures of (i) carboxylic acid, (ii) alcohol and (iii) the compound X. Also write the reaction. [2013]
Q.5. What is covalent bond? What type of bond exists in
(i) CCl4 (ii) CaCl2 (iii) CH4 (iv) NH3 [2013]
Q.6. Give the molecular formula and electron dot structure of ethyne and ethene. [2013]
Q.7. Draw the possible isomers of the compound with molecular formula C3H6O and also give their electron dot structures. [2013]
Q.8. Name the type of carbon compounds that can be hydrogenated. With the help of suitable example explain the process of hydrogenation. [2013]
Q.9. Name the product formed when an organic acid and alcohol react in the presence of an acid catalyst. Write the equation and give two uses of the product formed. [2013]
Q.10. Name the following compounds: [2013]
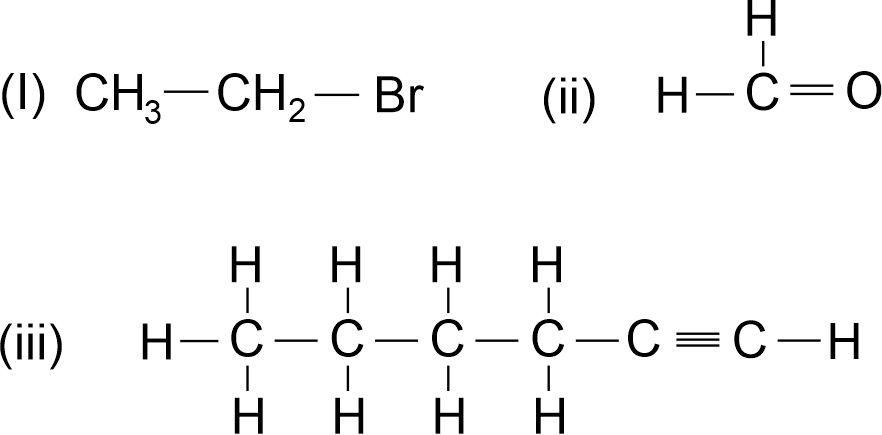
Q.11. Some esters are added to food items for special smells. An ester can be made from ethanol and ethanoic acid.
(i) Name the ester which is obtained due to the chemical reaction between ethanol and ethanoic acid in the presence of concentrated sulphuric acid and write the chemical equation.
(ii) Name the process. [2015]
Q.12. Ethyl ethanoate smells like pears and is used for flavouring sweets.
(i) Write the chemical formula of ethyl ethanoate.
(ii) Write the chemical reaction between ethanoic acid and ethanol in the presence of concentrated sulphuric acid.
(iii) Suggest the function of concentrated sulphuric acid in the reaction. [2015]
Q.13. On dropping a small piece of sodium in a test tube containing carbon compound X with molecular formula C2H6O, a bisk effervescence is observed and a gas Y is produced. On bringing a burning splinter at the mouth of the test tube the gas evolved burns with a pop sound. Identify X and Y. Also write the chemical equation for the reaction. Write the name and structure of the product formed, when you heat X with excess concentrated sulphuric acid. [2016]
Q.14. When ethanol reacts with ethanoic acid in the presence of conc. H2SO4, a substance with fruity smell is produced. Answer the following:
(i) State the class of compounds to which the fruity-smelling compounds belong. Write the chemical equation for the reaction and write the chemical name of the product formed.
(ii) State teh role of conc. H2SO4 in this reaction. [2016]
Q.15. Name the compound formed when ethanol is heated in excess of concentrated sulphuric acid at 443 K. Also write the chemical equation of the reaction stating the role of concentrated sulphuric acid in it. What would happen, if hydrogen is added to the product of this reaction in the presence of catalyst such as palladium or nickel? [2016]
Q.16. An aldehyde as well as a ketone can be represented by the same molecular formula, say C3H6O. Write the structures and name them. State the relation between the two in the language of science. [2016]
Q.17. (i) Why are most carbon-containing compounds poor conductors of electricity?
(ii) Write the name and structure of a saturated compound in which the carbon atoms are arranged in a ring. Give the number of single bonds present in this compound. [2018]
Q.18. What are soaps chemically? How do they differ from synthetic detergents? Also, mention their uses. [2019]
Q.19. Write the name and molecular formula of a carbon containing compounds having its name suffixed with “-ol” and having two carbon atoms in its molecule. With the help of a chemical equation indicate what happens, when this compound is heated with excess conc. H2SO4? [2019]
Q.20. In three test tubes A, B and C are three different liquids namely, distilled water, underground water and distilled water in which a pinch of calcium sulphate is dissolved, respectively are taken. Equal amount of soap solution is added to each test tube and the contents are shaken. In which test tube will the length of the foam (lather) be longest? Justify your answer. [2019]
Q.21. What happens, when 5% alkaline potassium permanganate solution is added drop by drop to warm propyl alcohol (propanol) taken in a test tube? Explain with the help of a chemical equation. [2019]
Q.22.”Conversion of ethanol to ethanoic acid is an oxidation reaction.” Justify this statement giving the relevant equation for the chemical reaction involved. [2019]
Q.23. Consider the molecular formula of the carbon compounds (i) and (ii) given below:
(i) C3H8O (ii) C3H6O2
(a) Identify the functional groups in (i) and (ii) and write their structures.
(b) Are (i) and (ii) isomers? Give reason.
(c) What happens when alkaline KMnO4 is added, drop by drop, into a test tube containing warm propanol? Write the chemical equation for the reaction and state the role of alkaline KMnO4 in this reaction. [2020]
Q.24. (a) Define isomerism. Draw all possible isomers of butane.
(b) “A compound ‘X’ on combustion gives a yellow flame with lots of smoke.” What inference would you draw from this statement?
(c) State the role of alkaline KMnO4 in the reaction involving conversion of an alcohol to corresponding carboxylic acid. [2020]
Q.25. (a) Carry out the following conversions:
(i) Ethanol to ethene
(ii) Ethanol to ethanoic acid
(b) Differentiate between addition reaction and substitution reaction. Give one example of each. [2020]
Long Questions
Q.1. (i) Write the names of the functional group in
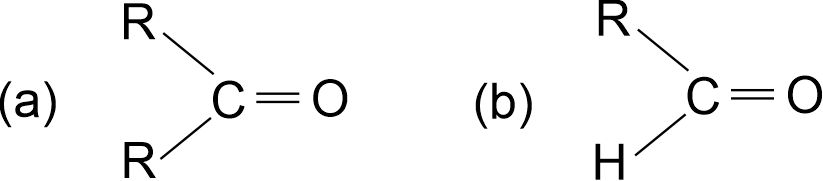
(ii) Describe a chemical test to distinguish between ethanol and ethanoic acid. [2009]
Q.2. (i) Complete the following reactions and name the main product formed in each case.
$$\mathrm{( a) \ CH_{3} CH_{2} CH_{2} OH\ \ +\ \ 2[ O] \ \xrightarrow[K_{2} Cr_{2} O_{7}]{Acidified} \ }$$
$$\mathrm{( b) \ C_{2} H_{5} COOH\ \ +\ \ NaHCO_{3} \ \longrightarrow \ }$$
$$\mathrm{( c) \ C_{3} H_{7} COO\ C_{2} H_{5} \ \ +\ \ NaOH\ \longrightarrow \ }$$
(ii) Write the names of the following compounds:
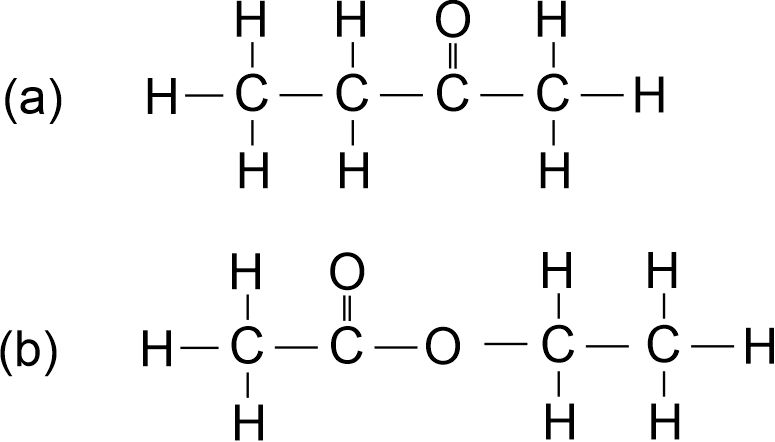
State the functional group present in each compound. [2011]
Q.3. An organic compound A is widely used as a preservative in pickles and has a molecular formula C2H4O2. This compound reacts with ethanol to form a sweet smelling compound B.
(i) Identify the compound A.
(ii) Write the chemical equation for its reaction with ethanol to form compound B.
(iii) How can we get compound A from B?
(iv) Name the process and write corresponding chemical equation.
(v) Which gas is produced when compound A reacts with washing soda? Write the chemical equation. [2011]
Q.4. Identify the compounds A to E in the following reaction sequence. [2012]
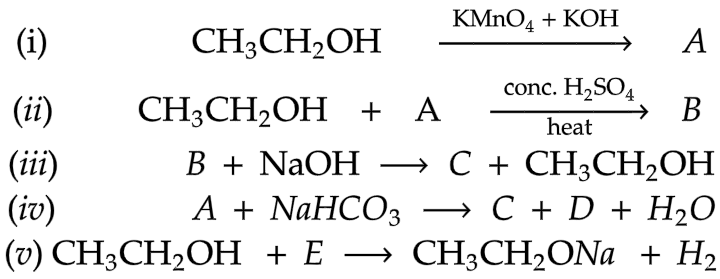
Q.5. (i) How is vinegar made?
(ii) What is glacial acetic acid? What is its melting point?
(iii) Why is butanoic acid a weak acid?
(iv) Write the name and the formula of the two compounds formed when the ester CH3COOC2H5 undergoes saponification. [2012]
Q.6. State the reason why carbon can neither form C4+ cations nor C4- anions but forms covalent compounds?
Also, state the reasons to explain why covalent compounds
(i) bad conductors of electricity?
(ii) have low melting and boiling points? [2014]
Q.7. Define structural isomer and draw the isomeric structures of butane. Compare the structure of benzene and cyclohexane by drawing them. [2015]
Q.8. Describe the addition reaction of carbon compounds with its application. State the function of catalyst in this reaction. How this reaction is different from a substitution reaction? Explain with an example. [2015]
Q.9. (i) Give a chemical test to distinguish between saturated and unsaturated hydrocarbon.
(ii) Name the products formed when ethane burns in air. Write the balanced chemical equation for the reaction showing the types of energies liberated.
(iii) Why is reaction between methane and chlorine in the presence of sunlight considered a substitution reaction? [2016]
Q.10. Write the chemical formula and name of the compound which is the active ingredient of all alcoholic drinks. List its two uses. Write chemical equation and name of the product formed when this compound reacts with
(i) sodium metal
(ii) Hot concentrated sulphuric acid. [2019]
Q.11. What is methane? Draw its electron dot structure. Name the type of bonds formed in this compound. Why are such compounds
(i) poor conductors of electricity?
(ii) have low melting and boiling points?
What happens, when this compound burns in oxygen? [2019]
Q.12. (i) Distinguish between esterification and saponification reactions with the help of chemical equations for each.
(ii) With a labelled diagram describe in brief an activity to show the formation of an ester. [2019]
Q.13. What is the difference between soaps and detergents? State in brief the cleansing action of soaps in removing an oily spot from a fabric. Why are soaps not very effective when a fabric is washed in hard water? How is this problem resolved? [2019]
Q.14. (i) What are isomers? Write the structures of two compounds having molecular formula C3H6O and give their names.
(ii) What are soaps? How are they chemically different from detergents? Why do soaps not work effectively in hard water? [2023]
Q.15. (i) What is a homologous series of carbon compounds? Write general formula for alkynes. Name and draw the electron dot structure of first homologue of this series.
(ii) State the meaning of the functional group in an organic compound. Write the formula of the functional group present in alcohols and carboxylic acids. [2023]
Q.16. (i) A compound ‘A’ with a molecular formula of C2H2O2 reacts with a base to give salt and water. Identify ‘A’, state its nature and the name of the functional group it possesses. Write chemical equation for the reaction involved.
(ii) When the above stated compound ‘A’ reacts with another compound ‘B’, having molecular formula C2H6O in the presence of an acid, a sweet smelling compound ‘C’ is formed.
(a) Identify ‘B’ and ‘C’.
(b) State the role of acid in this reaction.
(c) Write chemical equation for the reaction involved. [2023]
Q.17. (i) Name the compound formed when ethanol is heated at 443 K in the presence of conc. H2SO4 and draw its electron dot structure. State the role of conc. H2SO4 in this reaction.
(ii) What is hydrogenation? Explain it with the help of a chemical equation. State the role of this reaction in industry. [2023]
