Chapter – 2
Acids, Bases and Salts
2.1 Acids
Acids are those chemical substances that have a sour taste and change the colour of blue litmus to red.
For example, some common fruits such as unripe mango, orange, tamarind, lemon, etc., are sour in taste. So these fruits can be considered to contain acids.
In other words, an acid is a hydrogen containing compound which gives free hydronium ions [H3O+ or H+(aq)] when dissolved in water.
In the presence of water, all acids give H+ ion. As H+ ion cannot exist alone so it combines with water molecules and form H3O+ (hydronium ion). So, we can say that in presence of water, all acids give H+ ion or H3O+ ion.
HCl(g) + H2O (excess) → H3O+ (aq) + Cl– (aq)
H+ + H2O → H3O+
CH3COOH(aq) + H2O (excess) → H3O+ (aq) + CH3COO– (aq)
All acids conduct electricity in their aqueous solutions.
2.1.1 Classification of acids
Acids can be classified mainly into two groups depending upon sources:
(i) Inorganic acids or mineral acids: A mineral acid (or inorganic acid) is an acid derived from one or more inorganic compounds. All mineral acids form hydrogen ions (H+) and the conjugate base when dissolved in water. Most common inorganic acids are hydrochloric acid (HCl), nitric acid (HNO3) and sulphuric acid (H2SO4). These three acids are called bench acids. Mineral acids are strong acids.
Strong acids: The acids which are almost completely dissociated/ionised when dissolved in water are called strong acids. The solution of strong acids in water are highly conducting due to high concentration of hydrogen ion (H3O+ or H+) in the solution. The solutions of strong acids contain only ions.
Some examples of mineral acid in decreasing order of acidic strength
Mineral acids range from superacids (perchloric acid, HClO4) to very weak ones (boric acid).
Perchloric acid (HClO4), Hydroiodic acid (HI), Hydrobromic acid (HBr), Hydrochloric acid( HCl), Hydrofluoric acid (HF), Nitric acid (HNO3), Phosphoric acid (H3PO4,) Sulphuric acid (H2SO4), Boric acid (H3BO3), etc.
Mineral acids tend to be very soluble in water and insoluble in organic solvents.
These acids are corrosive in nature.
(ii) Organic acids: These acids include organic compounds belonging to carboxylic acid, sulphonic acid, and phenol. These are naturally occurring acids. These acids are weak.
Weak acids: The acids which are partially ionised when dissolved in water are called weak acids. The solution of weak acids in water is poor conducting due to low concentration of hydrogen ions (H3O+ or H+) in them. The solution of weak acids contains ions as well as molecules of the acid.
Concentrated acid and dilute acids
A solution that contains the maximum amount of the acidic gas (i.e., smaller amount of water) dissolved in it is called concentrated acid.
A solution that contains lesser amount of the acidic gas (i.e., larger amount of water) dissolved in it is called dilute acid.
The degree of dilution may vary with the amount of water added to the acid. Larger the amount of water added, more diluted the acid.
Some examples are
| Natural source | Acid | Natural source | Acid |
| Vinegar | Acetic acid | Sour milk (Curd) | Lactic acid |
| Orange | Citric acid | Lemon | Citric acid |
| Tamarind | Tartaric acid | Ant sting | Methanoic acid |
| Tomato | Oxalic acid | Nettle sting | Methanoic acid |
2.2 Base
Bases are those chemical substances which are bitter in taste, soapy in touch and turn red litmus blue. For example, Sodium hydroxide (NaOH), calcium hydroxide [Ca(OH)2], etc.
In other words, a base is a compound which gives free hydroxide ions (OH–) when dissolved in water. For example,
NaOH (aq) + H2O (excess) → Na+ (aq) + OH–(aq)
NH4OH(aq) + H2O (excess) → NH4+ (aq) + OH–(aq)
Ca(OH)2(aq) + H2O (excess) → Ca2+ (aq) + 2OH–(aq)
Note: All bases do not dissolve in water. An alkali is a base that dissolves in water. The alkalis in their aqueous solution conduct electricity. They are soapy to touch, bitter and corrosive. Never taste or touch them as they may cause harm.
2.2.1 Classification of bases
Strong Base: Bases which are highly soluble and completely dissociated/ionised in their solution in water are called strong bases. Strong bases are also called alkalis. For example, Sodium hydroxide (NaOH), and Potassium hydroxide (KOH). Generally, hydroxide of alkali metals (Li, Na, K, Rb, and Cs) are strong bases.
The solutions of strong bases (or alkalis) are highly conducting because they contain high concentration of OH– ions. The solutions of strong bases/alkalis contain only ions.
Note: Acidic nature of a substance is due to the formation of H+(aq) ions in solution. Formation of OH–(aq) ions in solution is responsible for the basic nature of a substance.
Weak Base: Bases which are partially ionised in their aqueous solutions are called weak bases. For example, Ammonium hydroxide (NH4OH), Calcium hydroxide [Ca(OH)2], Magnesium hydroxide [Mg(OH)2] etc.
The solutions of weak bases are very poorly conducting because they contain a very low concentrations of OH– ions. The solutions of weak bases contain ions as well as molecules of the bases.
2.3 Acid-base indicators
A substance that changes its colour when added to an acid or a base is called an acid-base indicator or simply an indicator. Acid-base indicators are dyes or mixtures of dyes that are used to indicate the presence of acids and bases.
Types of Indicators
(i) Natural Indicators: These indicators are found in nature in plants. For example, litmus solution, red cabbage leaves, turmeric, coloured petals of some flowers such as Hydrangea, Petunia and Geranium.
Note: Litmus solution is a purple dye, which is extracted from lichen, a plant belonging to the division of Thallophyta. When the litmus solution is neither acidic nor basic, its colour is purple.
The most commonly acid-base indicator used in school laboratories is litmus. Litmus is generally available in solution form and the form of paper strips such as Blue litmus and Red litmus.
(ii) Synthetic Indicators: The indicators which are synthesized in the laboratory or industry are known as synthetic indicators. For example, methyl orange, phenolphthalein, methylene blue, and methyl red.
(iii) Olfactory Indicators: Those substances whose odour changes in the acidic or basic medium are called olfactory indicators. For example, vanilla extract, clove and onion. The smell of these indicators can be detected in presence of acid only but not in the presence of a base.
(iv) Universal Indicators: A mixture of many common indicators each showing colour change over a certain pH values is commonly called the universal indicators. It has advantage because it shows different colours at different concentrations of hydrogen ions in a solution.
2.3.1 How to use of indicators
(i) Using indicator in solution form
- Take a small quantity of the given solution in a test tube or on a watch glass.
- Add 2-3 drops of the indicator solution into the sample solution with the help of a dropper.
- Observe colour of the solution.
- Match the colour change with the indicator colour chart. From the colour, find out if the solution is acidic, basic or neutral.
(ii) Using indicator in strip form
- Take a small piece of indicator strip.
- Put a drop of the given solution on it with the help of a dropper. Or, Dip an end of the indicator paper in the given solution.
- Match the colour change with the indicator colour chart. From the colour, find out if the solution is acidic, basic or neutral.
2.3.2 Colours of Indicators in acidic, basic and neutral solutions
| Indicators | Neutral Solution | Acidic Solution | Basic Solution | Remarks |
| Turmeric | Yellow | Yellow | Red | Indicator for bases |
| Red/Purple-cabbage | Purple/dark blue | Red or Pink | Green or Yellow | Indicator for acids as well as for bases |
| Hydrangea | Red or pink | Blue | Red or pink | Indicator for acids |
| Litmus | Purple-blue | Red | Blue | Indicator for acids as well as for bases |
| Phenolphthalein | Colourless | Colourless | Pink | Indicator for bases |
| Methyl orange | Yellow | Red | Yellow | Indicator for acids |
2.4 Chemical properties of Acids
2.4.1 Corrosive nature
Concentrated mineral acids such as sulphuric acid, and nitric acid attack vigorously human tissues, cloth, paper, and metals. Concentrated (conc.) sulphuric acid cuts through cloth and produces black spots on wood.
The natural acids attack metals slowly producing toxic compounds. That is why, citrus fruits, curds, etc., are never stored in utensils made of copper, lead, and zinc.
2.4.2 Reactions with metals
Acids react with most metals to produce the corresponding salts and give out hydrogen gas.
Acid + Metal → Salt + Hydrogen gas
For example,
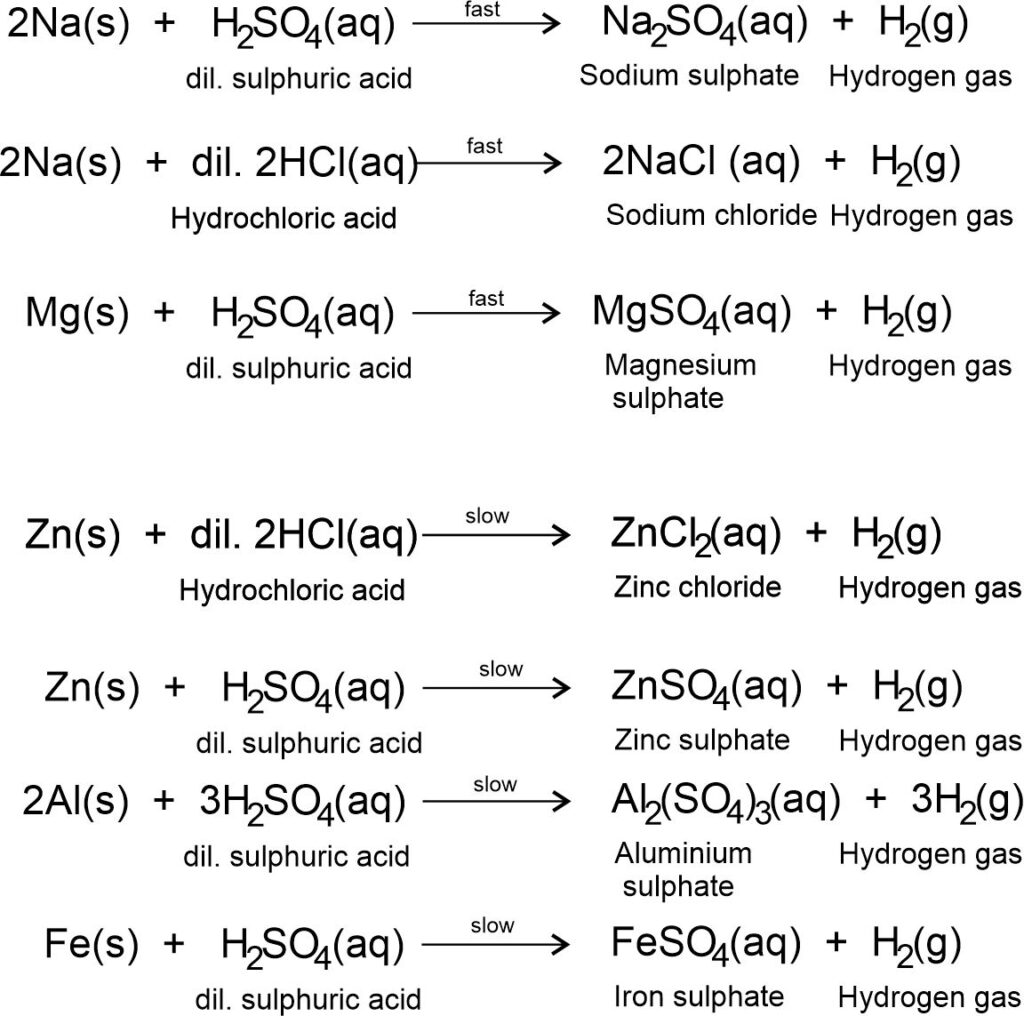
Metals in the above reactions displace hydrogen gas from acids. These reactions are displacement reactions. Here, metals mentioned in these reactions are more reactive than hydrogen.
The rate of reaction depends on the nature of metals and the strength of acids. For example,
Most reactive metals such as alkali metals i.e., Sodium (Na), Potassium (K), etc., and alkaline earth metals i.e., magnesium (Mg), Calcium (Ca), etc., react vigorously with dilute acids.
Moderate reactive metals such as Aluminium (Al), Zinc (Zn), iron (Fe), etc., react moderately with dilute acids.
Note: Metals do not react with weak acids such as carbonic acid (H2CO3). Metals react differently with concentrated acids.
Less reactive metals such as Copper (Cu), Silver (Ag), and Gold (Au), and noble metals such as Ruthenium (Ru), Rhodium (Rh), Palladium (Pd), Osmium (Os), Iridium (Ir), Platinum (Pt), do not react with dilute acids.
An experimental setup for the reaction of Zinc granule with dilute sulphuric acid

Test for H2 gas evolution
When a burning matchstick or candle is brought near the hydrogen gas, it burns with pop sound.
2.4.3 Reactions with metal carbonates and hydrogen carbonates
Acids react with metal carbonates and hydrogen carbonates (or bicarbonates) to produce their corresponding salts, carbon dioxide gas and water.
Acid + Metal carbonates → Salt + Carbon dioxide + water
For example, Sodium carbonate (washing soda) reacts hydrochloric acid to give sodium chloride, water and carbon dioxide gas.

Acid + Metal carbonates → Salt + Carbon dioxide + water
For example, Sodium hydrogen carbonate (baking soda) reacts hydrochloric acid to give sodium chloride, water and carbon dioxide gas.

An experimental setup for the reaction of sodium carbonate with hydrochloric acid
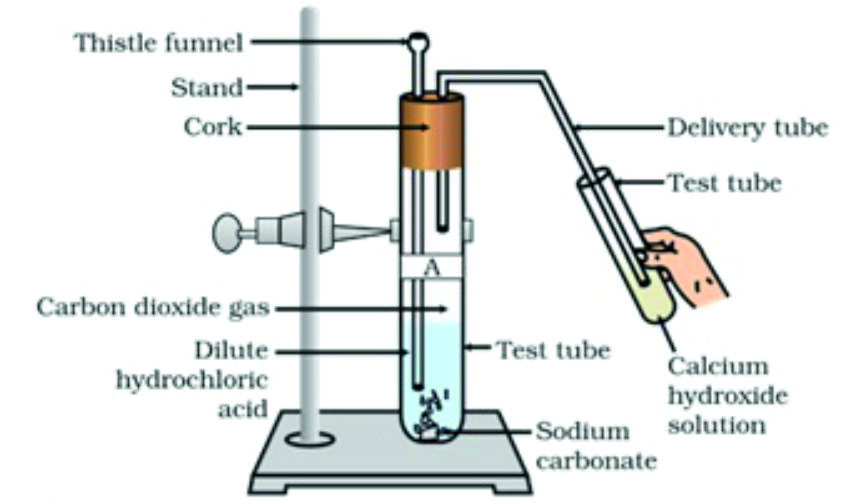
Test for CO2 gas
When CO2 gas is passed through lime water [Ca(OH)2], it turns milky due to the formation of white precipitate of CaCO3.
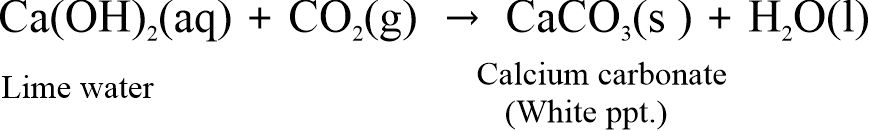
But, on passing excess CO2 gas, milkiness disappears due to the formation of calcium hydrogen carbonate Ca(HCO3)2 which is soluble in water.

2.4.4 Reactions with metal oxides
Metal oxides are basic in nature so they are also called basic oxides. Acids react with certain metal oxides to form salt and water.
Metal oxide + Acid → Salt + Water
For example,
(i) Metal oxides react with hydrochloric acid (HCl) to form corresponding metal chlorides and water.
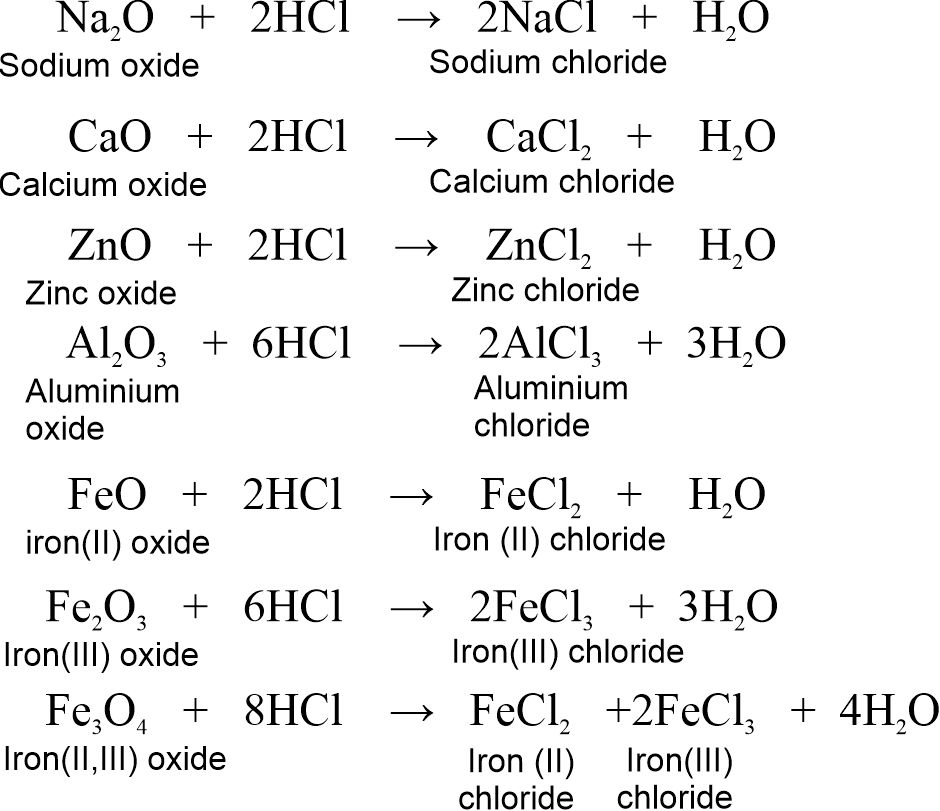
(ii) Metal oxides react with Sulphuric acid (H2SO4) to form corresponding metal sulphates and water.

(iii) Metal oxides react with nitric acid (HNO3) to form corresponding metal nitrate and water.

Note: Hematite (Fe2O3) and Magnetite (Fe3O4) are the two main ores of iron.
2.5 Chemical properties of Bases
2.5.1 Corrosive nature
Strong bases (alkalis) have corrosive effect on the skin. Sodium hydroxide breaks down fatty acids in the tissues and penetrates deeply and cause slow healing burns.
2.5.2 Reaction with metals
Metals being very weak bases themselves do not react with bases. Some metals like Zn and Al which are amphoteric react, with alkalis (strong bases) to produce corresponding salts and hydrogen gas.
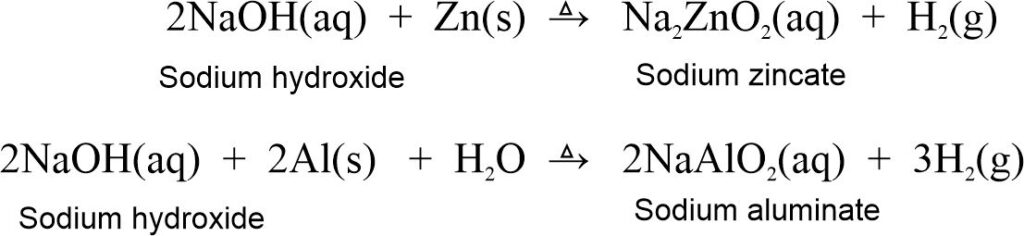
2.5.3 Reaction with non-metal oxides
Non-metal oxides are acidic in nature so they are also called acidic oxides. For example, CO2, SO2, etc. Non-metal oxides react with alkalis to produce salt and water.
Non-metal oxide + Base → Salt + Water
For example,

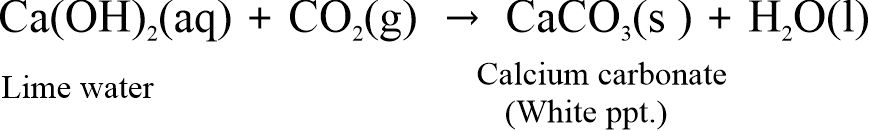
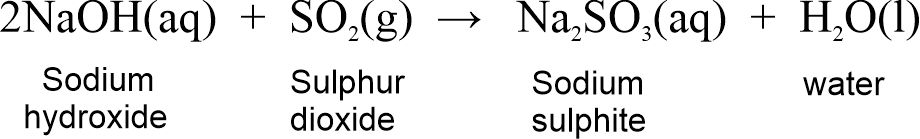
2.5.4 Action of heat
Some bases on heating lose water to form the corresponding oxides. For example,
(i) Magnesium hydroxide, on heating, loses water and forms magnesium oxide.
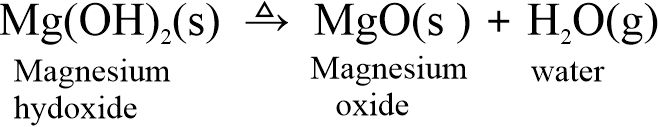
(ii) When ammonium hydroxide is heated, it produces ammonia and water.
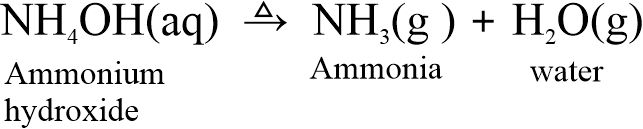
(iii) Aluminium hydroxide, on heating gives aluminium oxide and water.

(iv) Solid alkalis such as NaOH and KOH melt on heating but do not compose.
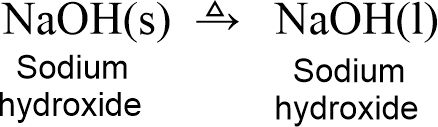
2.6.1 Reaction between Acids and Bases
Acids react with bases to produce salt and water. The reaction between an acid and a base in order to neutralise them i.e., an acid neutralises or reduces the effect of a base or vice-versa is called a neutralisation reaction. In general,
Acid + Base → Salt + Water
HX + MOH → MX + HOH
Here, X is halogen, M is metal and other have their representation.
For example,
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
or,
H+(aq) + OH–(aq) → H2O(l)
2.6.2 Effect of Dilution on an Acid or Base
Mixing an acid or base with water results in decrease in the concentration of ions (H3O+/OH–) per unit volume. Such a process is called dilution and the acid or the base is said to be diluted. When acid or base is added to water, their molecules dissociate to form ions.
HCl(l) + H2O(l) → H3O+(aq) + Cl– (aq)
or,
HCl(aq) → H+(aq) + Cl– (aq)
NaOH(aq) → Na+(aq) + OH–(aq)
Note: The addition of an acid or a base in water is a highly exothermic reaction. So, care must be taken while mixing concentrated nitric acid or sulphuric acid with water. The acid must always be added slowly to water with constant stirring. If water is added to a concentrated acid, the heat generated may cause the mixture to splash out and cause burns. The glass container may also break due to excessive local heating.
2.6.3 What do all acids and bases have in common?
Acid and base both produce ions in an aqueous solution. Acids dissociate to produce H+ or H3O+(cations) and A– ions (anions) while bases OH– ions (anions) and M+ (Cations). These free ions carry the electrical charge from one place to another, hence conducting electricity.
2.6.4 Uses of Acids and Bases
Uses of some common acids are:
| Hydrochloric acid (HCl) | (i) For removing deposits from inside the boilers. The process is called descaling. (ii) For the preparation of chlorides and chlorine gas. (iii) For cleaning iron sheets before galvanisation. (iv) For extracting glue from bones. (v) In textile industry for dyeing. (vi) For cleaning kitchen sink and sanitaryware. |
| Nitric acid (HNO3) | (i) For preparing fertilizers, explosives, dyes, and drugs (ii) In the refining of gold and silver. (iii) For cleaning gold and silver ornaments. |
| Sulphuric acid (H2SO4) | (i) For preparing fertilizers, detergents, plastics, and synthetic fibres. (ii) In petroleum industry for refining. (iii) In lead-acid batteries (vehicle batteries, inverter batteries etc.) as electrolytes. |
| Boric acid (H3BO3) | (i) As an antiseptic. (ii) For making eye-wash. |
| Citric acid (C6H8O7) | For food preservation. |
| Carbonic acid (H2CO3) | For making aerated (soft) drinks. |
| Acetic acid (CH3COOH) | (i) For making table vinegar. (ii) For making pickles. |
Uses of Some Common Bases are:
| Sodium hydroxide or caustic soda (NaOH) | (i) In soap and detergent industry. (ii) For the manufacture of rayon. (iii) In paper and pulp industry. (iv) For manufacturing other chemicals. |
| Calcium hydroxide or slaked lime [Ca(OH)2] | (i) For whitewashing. (ii) For the manufacture of bleaching powder. (iii) In leather industry. (iv) For neutralising acidity of the soil. (v) For softening hard water. (vi) As an insecticides and pesticides. |
| Magnesium hydroxide [Mg(OH)2] | (i) For preparing antacids. (ii) to treat occasional constipation in children and adults on a short-term basis |
| Aluminium hydroxide [Al(OH)3] | (i) For making antacids. (ii) In textile industry. |
| Ammonium hydroxide (NH4OH) | (i) As a cleaning agent. (ii) For preparing ammonium salts. (iii) For manufacturing fertilizers, nylon and nitric acid. |
| Sodium carbonate (Na2CO3) | (i) For washing clothes. (ii) For manufacturing glass, detergents, etc. |
| Sodium hydrogencarbonate (NaHCO3) | (i) For preparing baking powder. (ii) In fire extinguishers. |
2.7 Strength of an Acid or a Base
The strength of an acid or a base depends on the number of H+ ions or OH– ions produced by them respectively. Larger the number of H+ ions produced by an acid, stronger is the acid. Similarly, larger the number of OH– ions produced by a base, stronger is the base. Acids that give rise to more H+ ions are said to be strong acids, and acids that give less H+ ions are said to be weak acids. Similarly, bases that give rise to more OH– ions are said to be strong bases, and bases that give less OH– ions are said to be weak bases.
2.7.1 pH scale
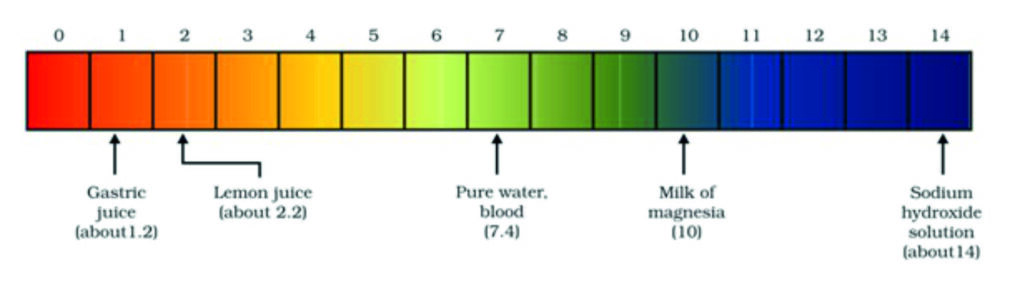
The strength of an acid or an alkali can be tested by using a scale called the pH scale (0-14) which gives the measure of hydrogen ion concentration in a solution.
The p in pH stands for ‘potenz’ in German, meaning power. On the pH scale we can measure pH from 0 (very acidic) to 14 (very alkaline). pH should be thought of simply as a number which indicates the acidic or basic nature of a solution. Higher the hydronium ion concentration, lower is the pH value.
The pH of a neutral solution is 7. Values less than 7 on the pH scale represent an acidic solution. As the pH value increases from 7 to 14, it represents an increase in OH– ion concentration in the solution, that is, increase in the strength of alkali. Generally paper impregnated with the universal indicator is used for measuring pH. One such paper is shown in figure below.


2.7.2 Importance of pH in Everyday Life
(i) Are plants and animals pH sensitive?
Our body works within the pH range of 7.0 to 7.8. Living organisms can survive only in a narrow range of pH changes. When pH of rainwater is less than 5.6, it is called acid rain. When acid rain flows into the rivers, it lowers the pH of the river water. The survival of aquatic life in such rivers becomes difficult.
Note – The atmosphere of Venus is made up of thick white and yellowish clouds of sulphuric acid.
(ii) What is the pH of the soil in your backyard?
Plants require a specific pH range for their healthy growth. Therefore, the nature of the soil is known first by testing its pH and then a particular crop is grown in it. It is suitable for selecting the fertiliser for a particular crop by knowing the pH of the soil.
(iii) pH in our digestive system
Our stomach produces hydrochloric acid. It helps in the digestion of food without harming the stomach. During indigestion, the stomach produces too much acid and this causes pain and irritation. To get rid of this pain, people use bases called antacids. These antacids neutralise the excess acid. Magnesium hydroxide (Milk of magnesia), a mild base, is often used for this purpose.
(iv) pH change as the cause of tooth decay
Tooth decay starts when the pH of the mouth is lower than 5.5. Tooth enamel, made up of calcium phosphate is the hardest substance in the body. It does not dissolve in water but is corroded when the pH in the mouth is below 5.5. Bacteria present in the mouth produce acids by degradation of sugar and food particles remaining in the mouth after eating. The best way to prevent this is to clean the mouth after eating food. Using toothpastes, which are generally basic, for cleaning the teeth can neutralise the excess acid and prevent tooth decay.
(v) Self-Defence by animals and plants through chemical warfare
When insects like honeybees, ant etc., bite, they inject an acid into the skin, that causes pain and irritation. If a mild base like baking soda is applied on the affected area, is given relief.
(vi) Nature provides neutralisation options
Nettle is a herbaceous plant which grows in the wild. Its leaves have stinging hair, which cause painful stings when touched accidentally. This is due to the methanoic acid secreted by them. A traditional remedy is rubbing the area with the leaf of the dock plant, which often grows beside the nettle in the wild.

2.8 Salts
Salts may be defined as the compound formed when neutralisation reaction takes placed between an acid and a base.
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
Here, HCl (an acid) reacts with NaOH (a base) to produce NaCl and water. Here, NaCl is a salt.
Other examples of salts are:
| Acids | Bases | Salts |
|---|---|---|
| Hydrochloric acid (HCl) | Sodium hydroxide (NaOH) | Sodium chloride (NaCl) |
| Hydrochloric acid (HCl) | Potassium hydroxide (KOH) | Sodium chloride (KCl) |
| Hydrochloric acid (HCl) | Calcium hydroxide [Ca(OH)2] | Calcium chloride [CaCl2] |
| Sulphuric acid (H2SO4) | Sodium hydroxide (NaOH) | Sodium sulphate (Na2SO4) |
| Sulphuric acid (H2SO4) | Potassium hydroxide (KOH) | Sodium sulphate (K2SO4) |
| Sulphuric acid (H2SO4) | Calcium hydroxide [Ca(OH)2] | Calcium sulphate (CaSO4) |
| Sulphuric acid (H2SO4) | Magnesium hydroxide [Mg(OH)2] | Magnesium sulphate (MgSO4) |
| Sulphuric acid (H2SO4) | Copper (ii) hydroxide [Cu(OH)2] | Copper sulphate (CuSO4) |
| Nitric acid (HNO3) | Sodium hydroxide (NaOH) | Sodium nitrate (NaNO3) |
| Nitric acid (HNO3) | Potassium hydroxide (KOH) | Potassium nitrate (KNO3) |
| Hydrochloric acid (HCl) | Ammonium hydroxide (NH4OH) | Ammonium chloride (NH4Cl) |
| Carbonic acid (H2CO3) | Sodium hydroxide (NaOH) | Sodium carbonate (Na2CO3) |
2.8.1 Family of salts
Salts having the same positive or negative radicals are said to belong to a family. For example, NaCl and Na2SO4 belong to the family of sodium salts. Similarly, NaCl and KCl belong to the family of chloride salts.
Some families of salts are:
| Sodium family (Na+) | Chloride family (Cl–) | Sulphate family |
|---|---|---|
| Sodium chloride (NaCl) Sodium carbonate (Na2CO3) Sodium bicarbonate (NaHCO3) Sodium sulphate (Na2SO4) Sodium nitrate (NaNO3) etc. | Sodium chloride (NaCl) Potassium chloride (KCl) Magnesium chloride (MgCl2) Aluminium Chloride (AlCl3) Ammonium chloride (NH4Cl) etc. | Sodium sulphate (Na2SO4) Potassium sulphate (K2SO4) Magnesium sulphate (MgSO4) Zinc sulphate (ZnSO4) Copper sulphate (CuSO4) etc. |
2.8.2 Classification of Salts
(i) Normal salts. When all the replaceable hydrogen atoms in an acid molecule are replaced by metal ions, the salt formed is called a normal salt.
When a strong acid reacts with a strong base, the salt formed is a normal salt. For example,
| Strong acids | Strong bases | Normal salts |
|---|---|---|
| Hydrochloric acid (HCl) | Sodium hydroxide (NaOH) | Sodium chloride (NaCl) |
| Hydrochloric acid(HCl) | Potassium hydroxide (KOH) | Potassium chloride (KCl) |
| Sulphuric acid (H2SO4) | Sodium hydroxide (NaOH) | Sodium sulphate (Na2SO4) |
| Sulphuric acid (H2SO4) | Potassium hydroxide (KOH) | Potassium sulphate (K2SO4) |
| Nitric acid (HNO3) | Sodium hydroxide (NaOH) | Sodium nitrate (NaNO3) |
| Nitric acid (HNO3) | Potassium hydroxide (KOH) | Potassium nitrate (KNO3) |
(ii) Acidic salts: The salt obtained by partial replacement of ionisable hydrogen atoms in an acid molecule by metal ions is called an acidic salt. For example,
| Strong acids | Strong bases | Acidic salts |
|---|---|---|
| Sulphuric acid (H2SO4) | Sodium hydroxide (NaOH) | Sodium hydrogensulphate (NaHSO4) |
| Phosphoric acid (H3PO4) | Sodium hydroxide (NaOH) | Sodium dihydrogenphosphate (NaH2PO4) |
When a strong acid reacts with a weak base, acidic salt is formed. For example,
| Strong acids | Weak base | Acidic salts |
|---|---|---|
| Hydrochloric acid(HCl) | Aluminium hydroxide [Al(OH)3] | Aluminium chloride (AlCl3) |
| Sulphuric acid (H2SO4) | Copper hydroxide [Cu(OH)2] | Copper sulphate (CuSO4) |
| Sulphuric acid (H2SO4) | Zinc hydroxide [Zn(OH)2] | Zinc sulphate (ZnSO4) |
| Hydrochloric acid(HCl) | Iron(III) hydroxide [Fe(OH)3] | Iron(III) chloride (FeCl3) |
(iii) Basic salts: The salt which contains one or more hydroxyl groups (coming from the base) along with the anion (coming from the acid) is called a basic salt. For example,
Lead hydroxychloride [Pb(OH)Cl], Calcium hydroxychloride [Ca(OH)Cl] etc.
When a strong base reacts with a weak acid, basic salt is formed. For example,
| Strong bases | Weak acids | Basic salts |
|---|---|---|
| Sodium hydroxide (NaOH) | Carbonic acid (H2CO3) | Sodium hydrogencarbonate (NaHCO3) |
| Sodium hydroxide (NaOH) | Acetic acid (CH3COOH) | Sodium ethanoate (CH3COONa) |
| Sodium hydroxide (NaOH) | Carbonic acid (H2CO3) | Sodium carbonate (Na2CO3) |
2.8.3 pH of salt solutions
Salts of a strong acid and a strong base are neutral with pH = 7.
For example, NaCl solution, Potassium nitrate (NaNO3) solution, etc.
Salts of a strong acid and weak base are acidic with pH < 7.
For example, Aluminium chloride (AlCl3) solution, Zinc sulphate (ZnSO4) solution, Copper sulphate (CuSO4) solution, etc.
Salts of a strong base and weak acid are basic in nature, with pH >7.
For example, Sodium acetate (CH3COONa) solution, Sodium carbonate (Na2CO3) solution, Sodium hydrogencarbonate (NaHCO3) solution, etc.
2.8.4 Chemicals from Common Salt
The salt formed by the combination of hydrochloric acid and sodium hydroxide solution is called sodium chloride. This is the salt that we use in food. It is a neutral salt.
Seawater contains many salts dissolved in it. Sodium chloride is separated from these salts. Deposits of solid salt are also found in several parts of the world. These large crystals are often brown due to impurities. This is called rock salt. Beds of rock salt were formed when seas of bygone ages dried up. Rock salt is mined like coal.

The common salt thus obtained is an important raw material for various materials of daily use, such as sodium hydroxide, baking soda, washing soda, bleaching powder, and many more.
1. Sodium hydroxide
Commercially, sodium hydroxide is also called caustic soda because of its corrosive action on animal and vegetable tissues. Its chemical formula is NaOH.
Preparation
When electricity is passed through an aqueous solution of sodium chloride (called brine), it decomposes to form sodium hydroxide. The process is called the chlor-alkali process because of the products formed– chlor for chlorine and alkali for sodium hydroxide.
2NaCl(aq) + 2H2O(l) → 2NaOH(aq) + Cl2(g) + H2(g)
Chlorine gas is given off at the anode, and hydrogen gas at the cathode. Sodium hydroxide solution is formed near the cathode. The three products produced in this process are all useful.
Different uses of H2, Cl2 and NaOH

2. Bleaching powder
The chemical formula of Bleaching powder is CaOCl2, or Ca(OCl)Cl named calcium oxychloride. It is also called chloride of lime.
Preparation
Bleaching powder is produced by the action of chlorine on dry slaked lime [Ca(OH)2].

Uses of bleaching powder
Bleaching powder is used –
(i) for bleaching cotton and linen in the textile industry,
(ii) for bleaching wood pulp in paper factories,
(iii) for bleaching washed clothes in laundry
(iv) as an oxidising agent in many chemical industries, and
(v) for disinfecting drinking water to make it free of germs.
3. Baking Soda
It is a mild non-corrosive base. Its chemical formula is NaHCO3 and its chemical name is Sodium hydrogencarbonate or Sodium bicarbonate.
Preparation
It can be prepared from common salt by the action of water, carbon dioxide and ammonia.
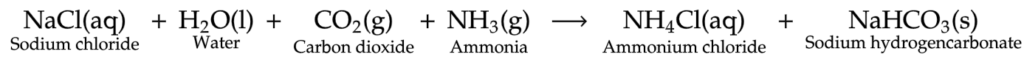
When it is heated, sodium carbonate is formed.

Uses of sodium hydrogencarbonate (NaHCO3)
(i) For making baking powder, which is a mixture of baking soda(sodium hydrogencarbonate) and a mild edible acid such as tartaric acid. When baking powder is heated or mixed in water, the following reaction takes place –

Carbon dioxide produced during the reaction causes bread or cake to rise making them soft and spongy. (ii) Sodium hydrogencarbonate is also an ingredient in antacids. Being alkaline, it neutralises excess acid in the stomach and provides relief.

(iii) It is also used in soda-acid fire extinguishers.
Note: Baking soda is also acts as a preservative for milk. In summer, it is added to the milk, as milk decompose and release lactic acid which makes milk sour. Added NaHCO3 reacts with acid to form salt and water. It neutralises the acidic effect adn milk does not become sour.
4. Washing soda
The chemical formula of washing soda is Na2CO3.10H2O and its chemical name is Sodium carbonate decahydrate.
Anhydrous sodium carbonate (Na2CO3) is generally called soda ash.
Preparation
Sodium carbonate can be obtained by heating baking soda. The recrystallization of sodium carbonate gives washing soda.

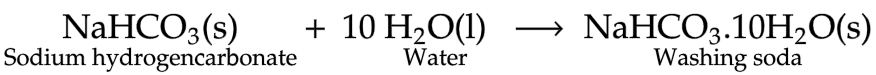
Uses of Washing soda
(i) Sodium carbonate (washing soda) is used in the glass, soap, and paper industries.
(ii) It is used in the manufacture of sodium compounds such as borax.
(iii) Sodium carbonate can be used as a cleaning agent for domestic purposes.
(iv) It is used for removing permanent hardness of water.
5. Plaster of Paris
The chemical formula of Plaster of Paris is CaSO4.1/2H2O and its chemical name is Calcium sulphate hemihydrate.
Preparation
It is obtained by heating gypsum (CaSO4.2H2O) at 373 K. At this temperature, gypsum loses water molecules and forms plaster of Paris.

Plaster of Paris is a white powder which doctors use as plaster for supporting fractured bones in the right position. On mixing with water, it changes to gypsum once again giving a hard solid mass.

Note: When gypsum is heated above 400 K, dead burnt plaster (anhydrous CaSO4) is obtained which does not have the property of hardening.
Uses of Plaster of Paris
Plaster of Paris is used –
(i) for making toys, materials for decoration, and for making surfaces smooth.
(ii) for sealing air gaps
(iii) for making blackboard chalk.
(iv) by doctor for joining the fractured bones at right position, i.e., for making plaster to support fractured bones.
2.9 Water of Crystallisation
The fixed number of water molecules present in one formula unit of a salt is called its water of crystallisation. For example,
Gypsum has chemical formula, CaSO4.2H2O, containing two water molecule and hence, it has two molecules of water of crystallisation.
Questions
Q.1. Why should curd and sour substances not be kept in brass and copper vessel?
Ans: Curd and sour substances contain acids which react with brass and copper to form certain salts that are poisonous in nature and can cause food poisoning. Hence, sour substances like curds, pickles, etc., should not be kept in brass and copper vessels.
Q.2. Why do HCl, HNO3, etc., show acidic characters in aqueous solutions while solutions of compounds like alcohol and glucose do not show acidic characters?
Ans: Acidic character of a compound is due to the presence of replaceable H+ ions, which are released in an aqueous solution and are responsible for acidic characters. Acids like HCl, HNO3, etc., has replaceable H+ ions, so they show acidic characters in aqueous solution while alcohol and glucose do not have replaceable H+ ions, so they do not show acidic characters in aqueous solution.
Q.3. Why does dry HCl gas not change the colour of dry litmus paper?
Ans: Dry HCl gas does not undergo ionisation and, therefore, does not contain H+ ions. That is why it does not change the colour of the dry litmus paper.
Q.4. Why does an aqueous solution of an acid conduct electricity?
Ans: Due to the presence of free ions (both cations H+ or H3O+ and anions of the acid) in an aqueous solution of the acid, it conducts electricity.
Q.5. While diluting an acid, why is it recommended that the acid should be added to water and not water to the acid?
Ans: The addition of an acid or a base in water is a highly exothermic reaction. So, care must be taken while mixing concentrated nitric acid or sulphuric acid with water. The acid must always be added slowly to water with constant stirring. If water is added to a concentrated acid, the heat generated may cause the mixture to splash out and cause burns. The glass container may also break due to excessive local heating.
Q.6. How is the concentration of hydronium ion (H3O+) affected when a solution of an acid is diluted?
Ans: When a solution of an acid is diluted, the concentration of H3O+ per unit volume decreases.
Q.7. How is the concentration of hydroxide ions (OH–) affected when an excess base is dissolved in a solution of sodium hydroxide?
Ans: Sodium hydroxide is a strong base. When the excess base is dissolved in the solution of NaOH, the concentration of hydroxide ions (OH–) per unit volume increases due to dissociation of NaOH as well as the other added base in aqueous solution.
Q.8. What affect does the concentration of H+ (aq) ions have on the nature of the solution?
Ans: Higher the number of H+ ions in a solution, more acidic is the solution.
Q.9. Do basic solutions also have H+ (aq) ions? If yes, then why are these basic?
Ans: Yes, basic solutions also contain H+ (aq) ions but the concentration of OH– ions is much larger than that of H+ ions. That is why, these solutions are basic.
Q.10. Why does distilled water not conduct electricity, whereas rain water does?
Ans: Distilled water does not contain any ions so it does not conduct electricity whereas rain water dissolves acidic oxides such as CO2, SO2, etc., to form free H+ ions. Hence, rain water conducts electricity.
Q.11. Why do acids not show acidic behaviour in the absence of water?
Ans: Acids produce ions only in their aqueous solutions which are responsible for acidic behaviour. That is why, they do not show acidic behaviour in the absence of water.
Q.12. Fresh milk has a pH of 6. How do you think the pH will change as it turns into curds? Explain.
Ans: During curd formation from milk, lactic acid is produced which makes it acidic. Hence, pH will decrease from 6.
Q.13. A milkman adds a very small amount of baking soda to fresh milk.
(i) Why does he shift the pH of the fresh milk from 6 to slightly alkaline?
(ii) What do you expect to observe when milk comes to boil?
(iii) Why does this milk take a longer time to set as a curd?
Ans: (i) Alkaline medium does not allow milk to turn sour easily.
(ii) When milk is about to boil, there must be more effervescence due to the presence of baking soda.
(iii) When milk is set to curd, the presence of alkali does not allow it to become acidic easily. Hence, this milk take a longer time to set as a curd.
Q.14. Plaster of Paris should be stored in moisture-proof containers. Explain why?
Ans: Plaster of Paris absorbs water and sets it into a hard mass. This makes the plaster of Paris unusable. That is why it should be stored in a moisture-proof container.
Previous year questions
Short Questions
Q.1. How the following substances will dissociate to produce ions in their solutions
(i) Hydrochloric acid
(ii) Nitric acid
(iii) Sulphuric acid
(iv) Sodium hydroxide
(v) Potassium hydroxide
(vi) Magnesium hydroxide [2014]
Q.2. 2 ml of sodium hydroxide solution is added to a few pieces of granulated zinc metal taken in a test tube. When the contents are warmed, a gas evolves which is bubbled through a soap solution before testing. Write the equation of the chemical reaction involved and the test to detect the gas. Name the gas which will be evolved when the same metal reacts with dilute solution of a strong acid. [2018]
Q.3. The pH of a salt used to make tasty and crispy pakoras is 14. Identify the salt and write a chemical equation for its formation. List its two uses.[2018]
Q.4. What is tooth enamel chemically? State the condition when it starts corroding. What happens when food particles left in the mouth after eating degrades? Why do doctors suggest use of tooth powder/toothpaste to prevent tooth decay? [2011]
Or, Tooth enamel is one of the hardest substances in our body. How does it undergo damage due to the eating of chocolates and sweets? What should we do to prevent it? [2010, 2012]
Q.5. (i) A chemical compound X is used in glass and soap industry. Identify the compound and give its chemical formula.
(ii) How many molecules of water of crystallisation are present in compound X?
(iii) How will you prepare the above compound starting from sodium chloride? Write all relevant equations involved in the process. [2015]
Q.6. During electrolysis of brine, a gas ‘G’ is liberated at anode. When this gas ‘G’ is passed through slaked lime, a compound ‘C’ is formed, which is used for disinfecting drinking water.
(a) Write formula of ‘G’ and ‘C’.
(b) State the chemical equation involved.
(c) What is the common name of the compound ‘C’? Give its chemical name. [2020]
Q.7. Answer the following:
(i) What happens when crystals of washing soda are left open in dry air?
(ii) Name the change that takes place. Which two industries are based on the use of washing soda?
(iii) With the help of balanced chemical equation, state the reaction that takes place when sodium hydrogencarbonate is heating during cooking. [2012]
Q.8. Identify the acid and base which form sodium hydrogen carbonate. Write chemical equation in support of your answer. State whether this compound is acidic, basic or neutral. Also, write its pH value. [2019]
Q.9. A white powder is used by doctors to support fractured bones.
(i) Write the name and chemical formula of the powder.
(ii) How is this powder prepared?
(iii) When this white powder is mixed with water, a hard solid mass is obtained. Write a balanced chemical equation for the change.
(iv) Give one more use of this white powder. [2019]
Q.10. How is plaster of Paris chemically different from gypsum? How can they be interconverted? Write two used of Plaster of Paris. [2016]
Q.11. A chemical compound ‘X’ is used in the soap and glass industry. It is prepared from brine.
(i) Write the chemical formula, chemical name, and chemical formula of ‘X’.
(ii) Write the equation involved in its preparation.
(iii) What happens when it is treated with water containing Ca or Mg salts? [2020]
Q.12. (a) A substance ‘X’ is used as a building material and is insoluble in water. When it reacts with dil. HCl, it produces a gas which turns lime water milky.
(i) Write the chemical name and formula of ‘X’.
(ii) Write chemical equations for the chemical reactions involved in the above statements. [2023]
Q.13. A metal ‘M’ on reacting with dilute acid liberates a gas ‘G’. The same metal also liberates gas ‘G’ when reacts with a base.
(i) Write the name of gas ‘G’.
(ii) How will you test the presence of this gas?
(iii) Write chemical equations for the reactions of the metal with (a) an acid and (b) a base.[2023]
Long Questions
Q.1. What is the water of crystallisation? Write the common name and chemical formula of a commercially important compound which has ten water molecules as water of crystallisation. How is this compound obtained? Write the chemical equation also. List any two uses of this compound. [2014]
Q.2. (i) Identify the acid and the base from which NaCl is obtained. Which type of salt is it? When is it called rock salt? How is rock salt formed?
(ii) Blue litmus is added to two test tubes A and B containing dilute HCl and NaOH solution respectively. In which test tube, a colour change will be observed? State the colour change and give its reason. [2019]
Q.3. What is observed when 2 mL of dilute hydrochloric acid is added to 1 g of sodium carbonate taken in a clean and dry test tube? Write chemical equation for the reaction involved. [2019]
Q.4 Write the main difference between an acid and a base. With the help of suitable example, explain the term neutralisation and the formation of
(a) acidic,
(b) basic, and
(c) neutral salts. [2019]
Q.5. (i) Salt ‘P’ commonly used in bakery products on heating gets converted into another salt ‘Q’ which itself is used for the removal of hardness of water and a gas ‘R’ is evolved. The gas ‘R’ when passed through freshly prepared lime water turns milky. Identify ‘P’, ‘Q’, and ‘R’, giving chemical equation for the justification of your answer.
(ii) A solution ‘X’ gives orange colour when a drop of it falls on pH paper, while another solution, ‘Y’ gives bluish colour when a drop of it falls on pH paper. What is the nature of both the solution? Determine the pH of solution ‘X’ and ‘Y’. [2019]
Q.6. Match the following pH values 1, 7, 10, 13 to the solution given below
- Milk of magnesia
- Gastric juice
- Brine
- Aqueous sodium hydroxide
Amit and Rita decided to bake a cake and added baking soda to the cake batter.
Explain with a balanced reaction, the role of the baking soda. Mention any other use of baking soda.
