Chapter – 3
Metals and Non-Metals
Till now, scientists have discovered 118 elements. Each element has its unique properties. Based on their properties, all of them, can be divided into two main groups, metals and non-metals. Apart from these, some elements show properties of both metals and non-metals. These are called metalloids.
Metals
Those elements (except hydrogen) which form cations (positive ions) by losing electrons are called metals. For example, sodium, calcium, iron, zinc, lead, gold, silver, etc.
Physical properties of Metals
(i) Physical State: Under normal conditions, all metals except mercury are solids. Mercury is liquid in room temperature.
(ii) Metallic lustre: Metals, in their pure state, have a shining surface. This property is called metallic lustre. Gold, silver, and platinum are used for jewellery due to their lustrous nature.
(iii) Hardness: Most of the metals are hard. The harness varies from metal to metal. Chromium is the hardest material on the earth. Osmium is hard enough to scratch glass. Some alkalis such as lithium, sodium, and potassium so soft they can easily be cut by sharp knife.
(iv) Malleability: The property of metal due to which a metal can be beaten into a thin sheet is called malleability. Most of the metals are malleable. Gold and Silver are the most malleable metals. These metals can be hammered into foil much thinner than the thinnest paper.
(v) Ductility: It is the property due to which a metal can be drawn into thin wires. All metals are not equally ductile. Gold, Silver, and Copper are among the most ductile metals. A wire of about 2 km length can be drawn from one gram of gold.
[Note: It is because of their malleability and ductility that metals can be given different shapes according to our needs.]
(vi) Good conductor of heat: Generally metals are good conductor of heat i.e., they permit heat to pass through them, except lead and mercury, which are poor conductor of heat. Copper and silver are the best conductor of heat. Steel, aluminium, copper, lead etc. are used to make cooking utensils due to their heating property.

(vii) Electrical conductivity: Metals allow electricity to pass through them. Conductivity varies with metals. Silver is the best conductor of electricity. Copper is the second best conductor of electricity. However, copper, and aluminiumare used to make electric wires. Tungsten is used to make filament of electric bulb.
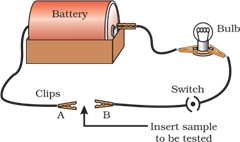
In the above setup, we have to insert different metals between terminals A and B to show their conducting nature.
(viii) Sonority: The metals that produce a sound on striking a hard surface are said to be sonorous. Due to this property, school bells are made up of metals. The wire strings in guitars, sitars, etc., produce musical sounds and are also made up of metals.
(ix) Tensile strength: Metals (except sodium, potassium, etc.) are very strong. They can bear a lot of stress. That is why the wheels of trains, hanging bridges, etc., are made of metals. Tungsten has the highest tensile strength of any pure metal – up to 500,000 psi at room temperature. It has the highest tensile strength even at temperatures over 1,500°C. Tungsten is so dense that it resists melting, even under extremely high heat.
(x) Melting point and Boiling point: Metals have high melting and boiling points. Tungsten has the highest melting point among metals, while gallium and caesium have very low melting points. These two metals will melt if we keep them on our palms.
Non-metals
Those elements which form negative ions by gaining electrons are called non-metals.
There are very few non-metals as compared to metals. There are approx. 22 non-metals. They are hydrogen (H), carbon(C), nitrogen (N), phosphorus (P), oxygen (O), sulphur (S), Selenium (Se), fluorine (F), chlorine (Cl), bromine (Br), iodine (I), Astatine (At), Tennessine (Ts), helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), redon (Rn) and Oganesson (Og).
Physical properties of non-metals
(i) Physical state: The non-metals are either solids or gases except bromine which is a liquid. Diamond, an allotrope of carbon, is the hardest known substance.
(ii) Brittleness: Solid non-metals are brittle. They break up into pieces when pressed hard or hammered. For example, black powder is made from coal which is carbon, a non-metal.
(iii) Malleability and ductility: Nonmetals are neither malleable nor ductile. Solid non-metals cannot be drawn into wires, and beaten into leaves/sheets because they are brittle.
(iv) Lustre: Non-metals do not have lustre. They do not shine. However, graphite (an allotrope of carbon) and iodine are the only non-metals which have lustre. So, non-metals cannot be polished.
(v) Electrical and thermal conductivity: Non-metals are generally poor conductors of heat and electricity. Graphite is the only non-metal which conducts electricity.
(vi) Sonority: Non-metals are non-sonorous. They do not produce rich sounds when hit with an object.
(vii) Allotropy: The property of an element to exist in more than one structural form is called allotropy. Different forms of an element are called allotropic forms or allotropes. For example,
(a) Carbon has different allotropes, coal, coke, lamp black, diamond, graphite, and Buckminsterfullerene etc.
(b) Phosphorus exists in five allotropes – white or yellow phosphorus, red phosphorus, violet phosphorus, black phosphorus and scarlet phosphorus.
(c) Sulphur has various allotropes – rhombic sulphur, monoclinic sulphur, plastic sulphur, etc.
(viii) Melting point and boiling point: Non-metals have low melting and boiling points. However, graphite has a high melting point (3700 0C). White phosphorus melts at 44 0C, and sulphur melts at 115 0C.
(ix) Tensile strength: Non-metals have low tensile strength. They can be easily broken.
(x) Density: Non-metals have usually low densities and are soft.
Conclusions
We conclude that we cannot group elements according to their physical properties alone, as there are many exceptions. For example –
(i) All metals except mercury exist as solids at room temperature. Metals have high melting points but gallium and caesium have very low melting points. These two metals will melt if we keep them on our palms.
(ii) Iodine is a non-metal but it is lustrous.
(iii) Carbon is a non-metal that can exist in different forms. Each form is called an allotrope. Diamond, an allotrope of carbon, is the hardest natural substance known and has a very high melting and boiling point. Graphite, another allotrope of carbon, is a conductor of electricity.
(iv) Alkali metals (lithium, sodium, potassium) are so soft that they can be cut with a knife. They have low densities and low melting points.
Elements can be more clearly classified as metals and non-metals on the basis of their chemical properties.
Chemical properties of metals
1. Reaction of metals with oxygen (Burning in air/oxygen)
Almost all metals combine with oxygen to form metal oxides.
Metal + Oxygen → Metal oxide
For example,
(a) when copper is heated in air, it combines with oxygen to form copper(II) oxide, a black oxide.
$$\mathrm{\underset{Copper}{2Cu( s)}} \ +\mathrm{\underset{Oxygen}{O_{2}( g)}} \ \xrightarrow{\triangle } \ \mathrm{\underset{Copper\ oxide}{2CuO( s)}}$$
(b) Aluminium forms aluminium oxide, when heated with in air.
$$\mathrm{\underset{Aluminium}{4Al( s)}} \ +\mathrm{\underset{Oxygen}{3O_{2}( g)}} \ \xrightarrow{\mathrm{\triangle}} \ \mathrm{\underset{Aluminium\ oxide}{2Al_2O_3( s)}}$$
(i) Generally, metals oxides are basic in nature. But, exceptionally, some metal oxides such as aluminium oxide (Al2O3), zinc oxide (ZnO) show both acidic and basic character., such metal oxides which react with both acids as well as bases to produce salt and water are called amphoteric acid. Aluminium oxide reacts in the following manner with acids and bases –
(a) Reaction with acid such as hydrochloric acid (HCl)
$$\mathrm{\underset{ \begin{array}{l}
Aluminium\ oxide\
( basic\ oxide)
\end{array}}{Al_{2} O_{3}( s)}} \ +\mathrm{\underset{Hydrochloric\ acid}{6HCl( aq)}} \ \rightarrow \ \mathrm{\underset{Aluminium\ chloride}{2AlCl_{3}( aq)}} +\ \ \mathrm{\underset{Water}{3\ H_{2} O( l)}}$$
(b) Reaction with base such as sodium hydroxide (NaOH)
$$\mathrm{\underset{ \begin{array}{l}
Aluminium\ oxide\
( basic\ oxide)
\end{array}}{Al_{2} O_{3}( s)}} \ +\mathrm{\underset{Sodium\ hydroxide}{2NaOH( aq)}} \ \rightarrow \ \mathrm{\underset{Sodium\ aluminate}{2NaAlO_{2}( aq)}} +\ \ \mathrm{\underset{Water}{\ H_{2} O( l)}}$$
(ii) Most metal oxides are insoluble in water but some of these dissolve in water to form alkalis. For example,
(a) Sodium oxide dissolve in water to produce sodium hydroxide.
$$\mathrm{\underset{Sodium\ oxide}{Na_2O( s)}} \ +\mathrm{\underset{Water}{H_{2}O( l)}} \ \xrightarrow \ \mathrm{\underset{Sodium\ hydroxide}{2NaOH( aq)}}$$
(b) Potassium oxide dissolves in water to produce potassium hydroxide.
$$\mathrm{\underset{Potassium\ oxide}{K_2O( s)}} \ +\mathrm{\underset{Water}{H_{2}O( l)}} \ \xrightarrow \ \mathrm{\underset{Potassium\ hydroxide}{2KOH( aq)}}$$
Note: all metals do not react with oxygen at the same rate. Different metals show different reactivities towards oxygen.
(a) Metals such as potassium and sodium react so vigorously that they catch fire if kept in the open. Hence, to protect them and to prevent accidental fires, they are kept immersed in kerosene oil.
(b) At ordinary temperature, the surfaces of metals such as magnesium, aluminium, zinc, lead, etc., are covered with a thin layer of oxide. The protective oxide layer prevents the metal from further oxidation.
(c) Iron does not burn on heating but iron filings burn vigorously when sprinkled in the flame of the burner.
(d) Copper does not burn, but the hot metal is coated with a black coloured layer of copper(II) oxide.
(e) Silver and gold do not react with oxygen even at high temperatures.
Hence, we can arrange the metals in order of reactivity towards oxygens as –
K > Na > Mg > Zn > Fe > Cu > Ag
Anodising
Anodising is a process of forming a thick oxide layer of aluminium. Aluminium develops a thin oxide layer when exposed to air. This aluminium oxide coat makes it resistant to further corrosion. The resistance can be improved further by making the oxide layer thicker. During anodising, a clean aluminium article is made the anode and is electrolysed with dilute sulphuric acid. The oxygen gas evolved at the anode reacts with aluminium to make a thicker protective oxide layer. This oxide layer can be dyed easily to give aluminium articles an attractive finish.
2. Reaction of Metals with water
Metal reacts with water and produce a metal oxide and hydrogen gas. Metal oxides that are soluble in water dissolves in it to further form metal hydroxide.
Metal + Water → Metal oxide + Hydrogen
Metal oxide + Water → Metal hydroxide
(i) All metals do not react with water.
(ii) Different metals react with water differently.
(a) Metals like potassium and sodium react violently with cold water. In case of sodium and potassium, the reaction is so violent and exothermic that the evolved hydrogen immediately catches fire.
$$\mathrm{\underset{Potassium}{2K( s)}} \ +\mathrm{\underset{Water}{2H_{2}O( l)}} \ \xrightarrow \ \mathrm{\underset{Potassium\ hydroxide}{2KOH( aq)}} \ +\mathrm{\underset{Hydrogen}{H_{2}( g)}}\ +heat\ energy$$
$$\mathrm{\underset{Sodium}{2Na( s)}} \ +\mathrm{\underset{Water}{2H_{2}O( l)}} \ \xrightarrow \ \mathrm{\underset{Sodium\ hydroxide}{2NaOH( aq)}} \ +\mathrm{\underset{Hydrogen}{H_{2}( g)}}\ +heat\ energy$$
[The heat evolved is sufficient for hydrogen to catch fire. That is why these metals are kept in ‘kerosene’ in order to avoid contact with both air and water.]
(b) The reaction of calcium with water is less violent. The heat evolved is not sufficient for the hydrogen to catch fire.
$$\mathrm{\underset{Calcium}{Ca( s)}} \ +\mathrm{\underset{Water}{2H_{2}O( l)}} \ \xrightarrow \ \mathrm{\underset{Calcium\ hydroxide}{Ca(OH)_{2}( aq)}} \ +\mathrm{\underset{Hydrogen}{H_{2}( g)}}$$
Calcium starts floating because the bubbles of hydrogen gas formed stick to the surface of the metal. These bubbles decrease the density of metal.
(c) Magnesium does not react with cold water. It reacts with hot water to form magnesium hydroxide and hydrogen.
$$\mathrm{\underset{Magnesium}{Mg( s)}} \ +\mathrm{\underset{Hot\ water}{2H_{2}O( l)}} \ \xrightarrow \ \mathrm{\underset{Magnesium\ hydroxide}{Mg(OH)_{2}( aq)}} \ +\mathrm{\underset{Hydrogen}{H_{2}( g)}}$$
Magnesium also starts floating due to the bubbles of hydrogen gas sticking to its surface.
(d) Metals like aluminium, iron and zinc do not react either with cold or hot water. But they react with steam to form the metal oxide and hydrogen.
$$\mathrm{\underset{Aluminium\ }{2Al( s)}} \ +\mathrm{\underset{Steam}{3H_{2} O( g)}} \ \rightarrow \ \mathrm{\underset{Aluminium\ oxide}{Al_{2} O_{3}( s)}} +\ \ \mathrm{\underset{Hydrogen}{3H_{2}( g)}}$$
$$\mathrm{\underset{Iron\ }{3Fe( s)}} \ +\mathrm{\underset{Steam}{4H_{2} O( g)}} \ \rightarrow \ \mathrm{\underset{Iron(II,III)\ oxide}{Fe_{3} O_{4}( s)}} +\ \ \mathrm{\underset{Hydrogen}{4H_{2}( g)}}$$
(e) Metals such as lead, copper, silver and gold do not react with water at all.
Thus, the reactivity order of metals toward water is
K > Na > Ca > Mg > Al > Fe > Pb > Cu > Ag > Au
3. Reaction of metals with acids
Metals react with acids to give salt and hydrogen gas.
Metal + Dilute acid → Salt + Hydrogen
All metals do not react with the acid in the same manner. The rate of formation of hydrogen gas bubbles shows the reactivity of metals towards dilute acid.
Except for a few very less reactive metals such as Cu, Hg, Ag, Au, Pt, etc., all metals react with dilute sulphuric acid and hydrochloric acid to produce salt and hydrogen gas. The decreasing rate of reactivity toward dilute acid is:
Mg > Al > Zn > Fe.
Note: Sodium and Potassium are not taken to react with dilute acid because they even react vigorously with cold water.
(i) Reaction of metals with dilute HCl
Metals react with dilute HCl to form metal chlorides (salt) and hydrogen gas. For example,
(a) Magnesium reacts with dilute HCl to form Magnesium chloride and hydrogen gas.
$$\mathrm{\underset{Magnesium\ }{Mg( s)}} \ +\mathrm{\underset{Dil.\ Hydrochloric\ acid}{2HCl( aq)}} \ \rightarrow \ \mathrm{\underset{Magnesium\ chloride}{MgCl_{2}( aq)}} +\ \ \mathrm{\underset{Hydrogen}{H_{2}( g) \uparrow }}$$
(b) Zinc reacts with dilute HCl to form Zinc chloride and hydrogen gas.
$$\mathrm{\underset{Zinc\ }{Zn( s)}} \ +\mathrm{\underset{Dil.\ Hydrochloric\ acid}{2HCl( aq)}} \ \rightarrow \ \mathrm{\underset{Zinc\ chloride}{ZnCl_{2}( aq)}} +\ \ \mathrm{\underset{Hydrogen}{H_{2}( g) \uparrow }}$$
(ii) Reaction of metals with dilute H2SO4
Metals react with H2SO4 to form Metal sulphate and hydrogen gas. For example,
(a) Magnesium reacts with dilute H2SO4 to form Magnesium sulphate and hydrogen gas.
$$\mathrm{\underset{Magnesium\ }{Mg( s)}} \ +\mathrm{\underset{Dil.\ Sulphuric\ acid}{H_{2}SO_{4}( aq)}} \ \rightarrow \ \mathrm{\underset{Magnesium\ sulphate}{MgSO_{4}( aq)}} +\ \ \mathrm{\underset{Hydrogen}{H_{2}( g) \uparrow }}$$
(b) Zinc reacts with with dilute H2SO4 to form zinc sulphate and hydrogen gas.
$$\mathrm{\underset{Zinc\ }{Zn( s)}} \ +\mathrm{\underset{Dil.\ Sulphuric\ acid}{H_{2}SO_{4}( aq)}} \ \rightarrow \ \mathrm{\underset{Zinc\ sulphate}{ZnSO_{4}( aq)}} +\ \ \mathrm{\underset{Hydrogen}{H_{2}( g) \uparrow }}$$
(iii) Reaction of metals with dilute HNO3 (nitric acid)
Hydrogen gas is not evolved when a metal reacts with nitric acid. It is because HNO3 is a strong oxidising agent. It oxidises the H2 produced to water and itself gets reduced to any of the nitrogen oxides (N2O, NO, NO2). But magnesium (Mg) and manganese (Mn) react with very dilute HNO3 to evolve H2 gas.
$$\mathrm{\underset{Magnesium\ }{Mg( s)}} \ +\mathrm{\underset{very\ dilute.\ nitric\ acid}{2HNO_{3}( aq)}} \ \rightarrow \ \mathrm{\underset{Magnesium\ nitrate}{Mg(NO_{3})_{2}( aq)}} +\ \ \mathrm{\underset{Hydrogen}{H_{2}( g) \uparrow }}$$
[Aqua regia, (Latin for ‘royal water’) is a freshly prepared mixture of concentrated hydrochloric acid and concentrated nitric acid in the ratio of 3:1. It can dissolve gold, even though neither of these acids can do so alone. Aqua regia is a highly corrosive, fuming liquid. It is one of the few reagents that is able to dissolve gold and platinum.]
(iv) Reaction of metals with solution of other metal salts
Reactive metals can displace a comparatively less reactive metal from its salt solution. The general formula is
Metal A + Salt solution of B → Salt solution of A + Metal B
For example,
$$\mathrm{\underset{Iron\ }{Fe( s)}} \ +\mathrm{\underset{Copper\ sulphate}{CuSO_{4}( aq)}} \ \rightarrow \ \mathrm{\underset{Iron\ sulphate}{FeSO_{4}( aq)}} +\ \ \mathrm{\underset{Copper}{Cu( s)}}$$

Reactivity Series
The reactivity series is a list of metals arranged in the order of their decreasing activities. Based on their relative tendency to lose an electron and their reactive nature, metals are arranged in a series, this series is called the activity series or reactivity series of metals.
The metals that are placed above hydrogen are called most reactive metals such as K, Na, Ca, etc. and the metals that are placed below hydrogen are called least reactive metals such as noble metals, i.e., gold, and platinum.
The metals which are more electropositive than hydrogen can displace hydrogen from dilute acids. For example, K, Ca, Zn, Pb, Fe, etc.
$$\mathrm{\underset{Zinc\ }{Zn( s)}} \ +\mathrm{\underset{Sulphuric\ acid}{H_{2}SO_{4}( aq)}} \ \rightarrow \ \mathrm{\underset{Zinc\ sulphate}{ZnSO_{4}( aq)}} +\ \ \mathrm{\underset{Hydrogen}{H_{2}( g)}}$$
The metals which are less electropositive than hydrogen can not displace hydrogen from dilute acids. For example, Cu, Ag, Hg, etc.
$$\mathrm{\underset{Copper\ }{Cu( s)}} \ +\mathrm{\underset{Sulphuric\ acid}{H_{2}SO_{4}( aq)}} \ \rightarrow \ \mathrm{no\ reaction}$$
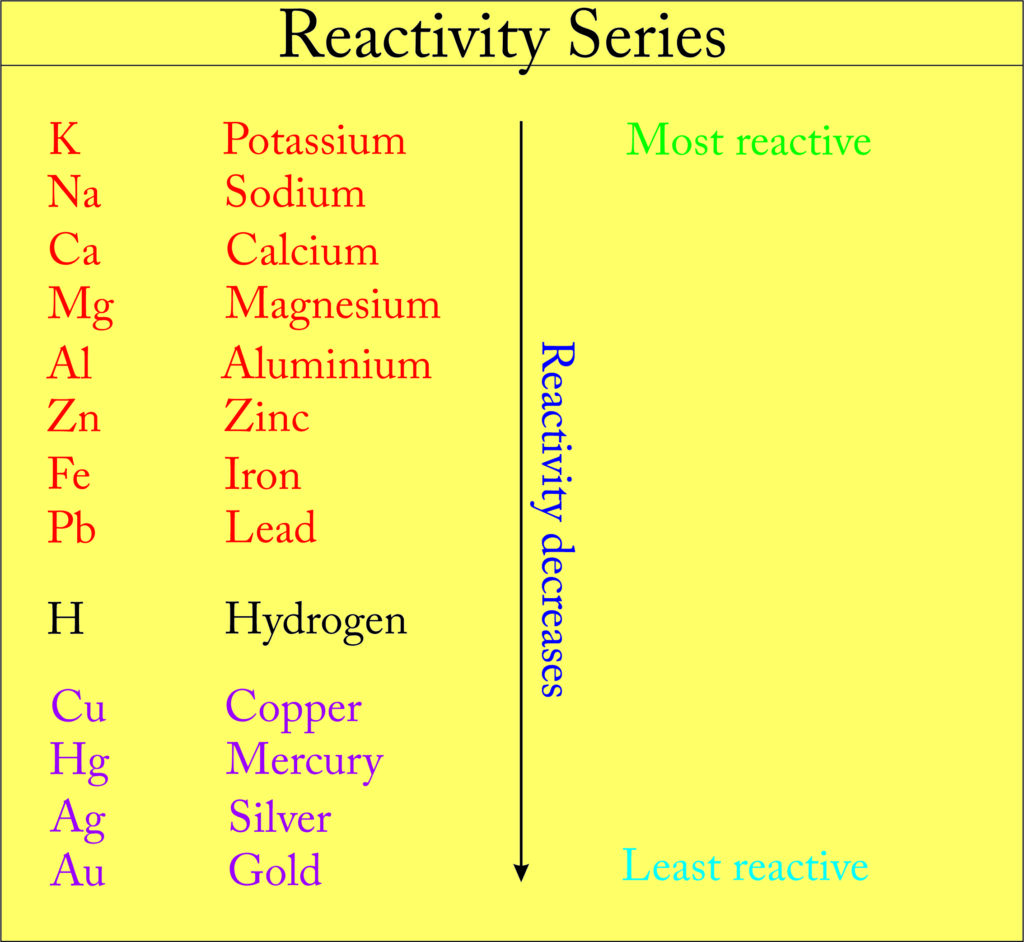
Note: Hydrogen also has non-metallic properties but, due to electropositive nature, it has been placed in the reactivity series. Metals above the hydrogen are more reactive than hydrogen and metals below the hydrogen are less reactive than hydrogen.
Chemical properties of non-metals
1. Non-metals do not react with water, steam, or dilute acids to evolve hydrogen gas because they act as an electron acceptor and cannot supply electrons to the H+ ions of acids to reduce them to hydrogen gas.
2. Heating with conc. acids: Non-metals readily form oxides of salts on heating with conc. acids. For example,
$$\mathrm{\underset{Sulphur}{S( s)}} \ +\mathrm{\underset{Conc.\ Sulphuric\ acid}{2H_{2} SO_{4}}} \ \xrightarrow{\mathrm{heat}} \ \mathrm{\underset{Sulphur\ dioxide}{3SO_{2}( g)}} +\ \ \mathrm{\underset{Water}{2H_{2} O( l)}}$$
$$\mathrm{\underset{Sulphur}{S( s)}} \ +\mathrm{\underset{Conc.\ nitric\ acid}{6HNO_{3}}} \ \xrightarrow{\mathrm{heat}}\mathrm{\underset{Sulphuric\ acid}{H_{2} SO_{4}( aq)}} \ \mathrm{\underset{Nitric\ acid\ ( Redish\ brown)}{6NO_{2}( g)}} +\ \ \mathrm{\underset{Water}{2H_{2} O( l)}}$$
3. Reaction with oxygen: Non-metals react with oxygen to give oxides. The oxides of non-metals may be acidic or neutral. The acidic oxides of non-metals when dissolved in water give acids. Thus, acidic oxides of non-metals turn blue litmus red. For example,
(i) Reaction of Carbon with oxygen
(a) In excess of oxygen: Carbon burns completely in the excess oxygen to give carbon dioxide gas. The carbon dioxide produced is acidic so it dissolves in water to give carbonic acid.
$$\mathrm{\underset{Carbon\ \ }{C( s)}} \ +\mathrm{\underset{Oxygen\ ( in\ excess)}{O_{2}( g)}} \ \mathrm{\xrightarrow{burn}} \ \mathrm{\underset{Carbon\ dioxide}{CO_{2}( g)}}$$
$$\mathrm{\underset{Carbon\ dioxide}{CO_{2}( g)}} +\ \ \mathrm{\underset{Water}{H_{2} O( l)}} \ \longrightarrow \ \mathrm{\underset{Carbonic\ acid}{H_{2} CO_{3}( aq)}}$$
(b) In limited oxygen: Carbon burns partially in the limited amount of oxygen to give carbon monoxide gas. Carbon monoxide gas being neutral, has no effect on litmus paper.
$$\mathrm{\underset{Carbon\ \ }{2C( s)}} \ +\mathrm{\underset{Oxygen\ ( in\ limited)}{O_{2}( g)}} \ \mathrm{\xrightarrow{burn}} \ \mathrm{\underset{Carbon\ dioxide}{2CO( g)}}$$
(ii) Reaction of Sulphur with oxygen
Sulphur burns in the presence of oxygen to yield sulphur dioxide. Sulphur dioxide, being an acidic oxide, gives sulphurus acid when dissolved in water.
$$\mathrm{\underset{Sulphur\ \ }{S( s)}} \ +\mathrm{\underset{Oxygen\ }{O_{2}( g)}} \ \mathrm{\xrightarrow{burn}} \ \mathrm{\underset{Sulphur\ dioxide}{SO_{2}( g)}}$$
$$\mathrm{\underset{Sulphur\ dioxide}{SO_{2}( g)}} +\ \ \mathrm{\underset{Water}{H_{2} O( l)}} \ \longrightarrow \ \mathrm{\underset{Sulphurus\ acid}{H_{2} SO_{3}( aq)}}$$
(iii) Reaction of hydrogen with oxygen
Hydrogen reacts with water to give water. Water is a neutral oxide. It has no effect on litmus.
$$\mathrm{\underset{Hydrogen\ \ }{H_{2}( g)}} \ +\mathrm{\underset{Oxygen\ }{O_{2}( g)}} \ \mathrm{\rightarrow } \ \mathrm{\underset{Water}{H_{2} O( l)}}$$
4. Reaction of non-metals with chlorine
Non-metals react with chlorine to give covalent chlorides. For example,
Hydrogen reacts with chlorine in the presence of diffused sunlight to form hydrogen chloride.
$$\mathrm{\underset{Hydrogen\ \ }{H_{2}( g)}} \ +\mathrm{\underset{\ Chlorine}{Cl_{2}( g)}} \ \mathrm{\xrightarrow[diffused]{sunlight}} \ \mathrm{\underset{Hydrogen\ chloride\ gas}{2HCl( g)}}$$
Hydrogen chloride gas is a polar covalent compound. It dissolves in water to give hydrochloric acid.
$$\mathrm{\underset{Hydrogen\ chloride\ gas}{HCl( g)}} \ \ \mathrm{\xrightarrow{H_{2} O}} \ \ \ \mathrm{\underset{Hydronium\ ion}{H_{3} O^{+}}} +\ \mathrm{\underset{Chloride\ ion}{Cl^{-}}} \equiv \ \mathrm{\underset{Hydrochloric\ acid}{HCl( aq)}}$$
5. Reaction of non-metals with hydrogen
Different non-metals react with hydrogen to form covalent compounds. For example,
(i) When hydrogen gas is passed through boiling sulphur, hydrogen sulphide gas is produced. Hydrogen sulphide solution turns blue litmus red.
$$\mathrm{\underset{Hydrogen\ \ }{H_{2}( g)}} \ +\mathrm{\underset{\ Sulphur\ ( Boiling)}{S}} \ \longrightarrow \ \mathrm{\underset{Hydrogen\ sulphide\ gas}{H_{2} S( g)}}$$
$$\mathrm{\underset{Hydrogen\ sulphide\ gas}{H_{2} S( g)}} \ \ \mathrm{\xrightarrow{H_{2} O}} \ \ \ \mathrm{\underset{}{H_{2} S( aq)}} \ \ \longrightarrow \ \mathrm{\underset{turns\ blue\ litmus\ red}{2H^{+} \ \ +\ \ S^{2-}}}$$
(ii) Nitrogen reacts with hydrogen to give ammonia, which is a basic in nature. When it dissolves in water, ammonium hydroxide (a base) is produced.
$$\mathrm{\underset{Hydrogen\ \ }{2H_{2}( g)}} \ +\mathrm{\underset{\ Nitrogen}{N_{2}( g)}} \ \longrightarrow \ \mathrm{\underset{Ammonia\ }{NH_{3}( g)}}$$
$$\mathrm{\underset{Ammonia\ }{NH_{3}( g)} \ \ +\ \ \underset{Water}{H_{2} O( l)} \ \ } \ \longrightarrow \ \ \ \mathrm{\underset{Ammonium\ hydroxide\ }{NH_{4} OH( g)}}$$
(iii) Carbon reacts with hydrogen in electric arc to give methane ( a neutral compound). It has no effect on litmus.
$$\mathrm{\underset{Hydrogen\ \ }{2H_{2}( g)}} \ +\mathrm{\underset{}{C( s)}} \ \xrightarrow{\mathrm{electric\ arc}} \ \mathrm{\underset{Methane\ }{CH_{4}( g)}}$$
6. Displacement reaction:
Non-metals also show displacement reaction like metals. For example,
When chlorine gas is passed through liquid sodium bromide, sodium chloride and bromine gas is produced.
$$\mathrm{\underset{Chlorine}{Cl_{2}( g)}} \ +\mathrm{\underset{Sodium\ bromide}{2NaBr( l)}} \ \ \longrightarrow \mathrm{\underset{Sodium\ chloride}{\ \ 2NaCl( l)}} \ +\ \ \mathrm{\underset{Bromine}{Br_{2}( g)}}$$
Reaction between metals and non-metals
Metal reacts with non-metal to form ionic compound. Each elements wants to have a completely filled valence shell, i.e., it want to have either 2 or 8 electrons in their outermost shell. Metals have tendency to loose electrons to form cations (+ve ions) and non-metals have tendency to gain electrons to form anions (-ve ions). The cation and anion so formed attract each other due to coulombic force of attraction and an ionic bond is formed. Thus,
An ionic bond is formed when one or more electrons are transferred from the metal atom to non-metal atom.
An ionic bond may also be defined as “The chemical bond formed by a complete transfer of one or more electrons from atom of a metal to that of a non-metal is called an ionic bond.” Such compounds formed are called ionic compounds.
Reactivity of elements:
Noble gases, which have a completely filled valence shell, show little chemical activity. We, therefore, explain the reactivity of elements as a tendency to attain a completely filled valence shell.
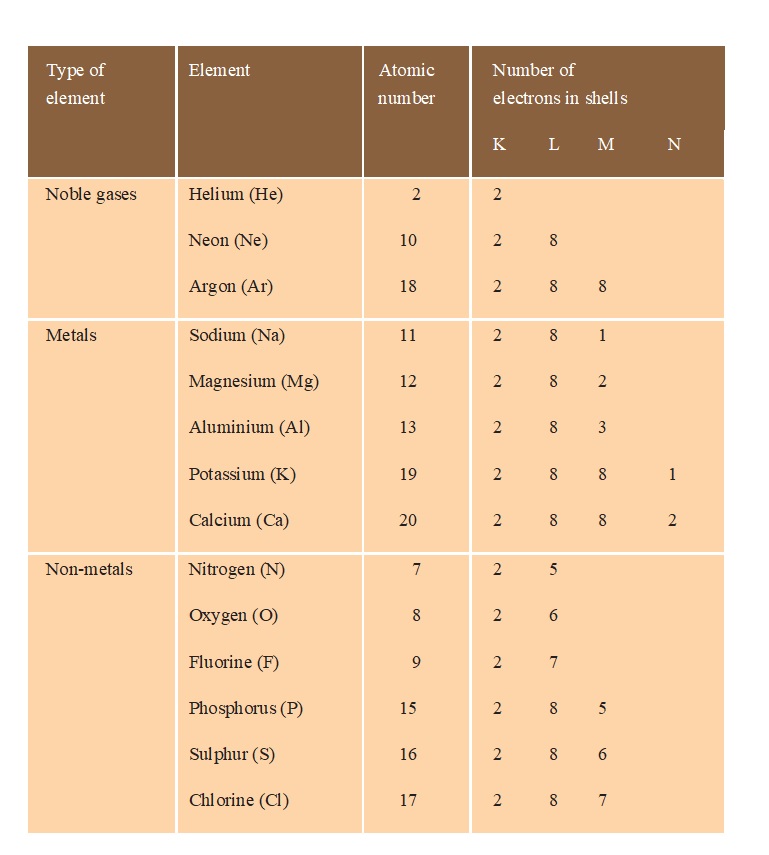
Electronic configuration of some elements
Formation of Ionic compounds:
(i) Formation of sodium chloride (NaCl)
(a) Formation of sodium ion: A sodium atom has one electron in its outermost shell. If it loses the electron from its M shell then its L shell now becomes the outermost shell and that has a stable octet. The nucleus of this atom still has 11 protons but the number of electrons has become 10, so there is a net positive charge giving us a sodium cation Na+.
$$\underset{\mathrm{Sodium}}{\mathrm{\underset{2,\ 8,\ 1}{Na}}} \ \ \longrightarrow \ \underset{\mathrm{Sodium\ ion\ ( cation)}}{\mathrm{\underset{2,\ 8}{\ Na^{+}}}} \ +\ \mathrm{e^{-}}$$
(b) Formation of chloride ion: A chlorine atom has seven electrons in its outermost shell and it requires one more electron to complete its octet. If sodium and chlorine were to react, the electron lost by sodium could be taken up by chlorine. After gaining an electron, the chlorine atom gets a unit negative charge, because its nucleus has 17 protons and there are 18 electrons in its K, L and M shells. This gives us a chloride anion C1–.
$$\mathrm{\underset{Chlorine}{\mathrm{\underset{2,\ 8,7}{Cl}}} \ } +\ \mathrm{e^{-}} \ \ \longrightarrow \ \underset{\mathrm{Chloride\ ion\ ( anion)}}{\mathrm{\underset{2,\ 8,\ 8}{\ Cl^{-}}}}$$
(c) Formation of Na+Cl– or NaCl: Sodium and chloride ions, being oppositely charged, attract each other and are held by strong electrostatic forces of attraction to exist as sodium chloride (NaCl).
$$\underset{\mathrm{Sodium\ ion\ ( cation)}}{\mathrm{\underset{2,\ 8}{\ Na^{+}}}} +\ \ \ \underset{\mathrm{Chlorine\ ion\ ( anion)}}{\mathrm{\underset{2,\ 8,\ 8}{\ Cl^{-}}}} \longrightarrow \ \ \ \ \ \mathrm{\underset{}{Na^{+} Cl^{-}}} \ \ \ or\ \ \ \mathrm{\underset{Sodium\ chloride}{NaCl}}$$
Electron dot structure of formation of NaCl

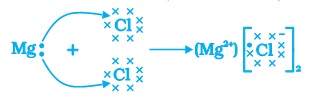
(ii) Formation of magnesium chloride (MgCl2)
A magnesium atom has two electrons in its valence shell and it loses two electrons to get an octet state. The chlorine atom has seven valence electrons and it gains one electron to get an octet state. So, one atom of magnesium will transfer its two valence electrons to two chlorine atoms, (one of each) in order to form magnesium chloride.
(a) Formation of magnesium ion
$$\mathrm{\underset{\mathrm{Magnesium}}{\mathrm{\underset{2,\ 8,\ 2}{Mg}}} \ \ \longrightarrow \ \underset{Magnesium\mathrm{\ ion\ ( cation)}}{\mathrm{\underset{2,\ 8}{\ Mg^{2+}}}} \ +\ 2e^{-}}$$
(b) Formation of chloride ion
$$\mathrm{\underset{Chlorine}{\mathrm{\underset{2,\ 8,7}{Cl}}} \ } +\ \mathrm{e^{-}} \ \ \longrightarrow \ \underset{\mathrm{Chloride\ ion\ ( anion)}}{\mathrm{\underset{2,\ 8,\ 8}{\ Cl^{-}}}}$$
(c) Formation of magnesium chloride

Electron dot structure of formation of MgCl2
Note: Ionic compound does not exist as molecules but aggregates of oppositely charged ions.
Physical properties of ionic compounds
1. Physical nature: Ionic compounds are solids and are somewhat hard because of the strong force of attraction between the positive and negative ions. These compounds are generally brittle and break into pieces when pressure is applied.
2. Melting and Boiling points: Ionic compounds have high melting and boiling points. This is because a considerable amount of energy is required to break the strong inter-ionic attraction.


3. Solubility: Electrovalent compounds are generally soluble in water and insoluble in solvents such as kerosene, petrol, etc.
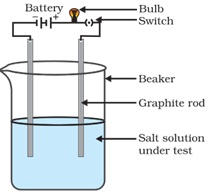
4. Conduction of Electricity: The conduction of electricity through a solution involves the movement of charged particles. A solution of an ionic compound in water contains ions, which move to the opposite electrodes when electricity is passed through the solution. Ionic compounds in the solid state do not conduct electricity because movement of ions in the solid is not possible due to their rigid structure. But ionic compounds conduct electricity in the molten state. This is possible in the molten state since the elecrostatic forces of attraction between the oppositely charged ions are overcome due to the heat. Thus, the ions move freely and conduct electricity.
Occurrence of Metals
Earth’s crust is the main source of metal. Sea water is also a source of many metals in the form of soluble salts.
Metals occur in nature in the free as well as in the combined states.
The less reactive metals like silver, gold, copper and platinum occur in the free state because they do react with water, air, etc. Thus, all metals which are not affected by water and by the gases present in the air occur in a free state in nature.
All metals which react with water, air, and other chemicals present in nature, occur in the combined state as compounds. For example,
Copper and silver are also found in the combined state as their sulphide or oxide ores. The metals at the top of the activity series (K, Na, Ca, Mg and Al) are so reactive that they are never found in nature as free elements. sodium metal in seawater as sodium chloride, calcium metal in marble as calcium carbonate, etc. The metals in the middle of the activity series (Zn, Fe, Pb, etc.) are moderately reactive. They are found in the earth’s crust mainly as oxides, sulphides or carbonates.
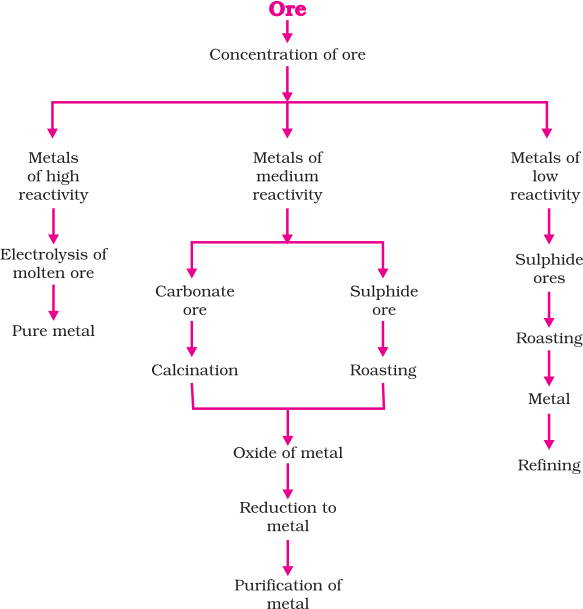
Thus on the basis of reactivity, we can group the metals into the following three categories and different techniques are to be used for obtaining the metals falling in each category.
(i) Metals of low reactivity – by electrolysis
(ii) Metals of medium reactivity – by reduction using carbon
(iii) Metals of high reactivity – found in native state (need not to be purified)
Different techniques are to be used for obtaining the metals falling in each category
| K Na Ca Mg Al | Electrolysis |
| Zn Fe Pb Cu | Reduction using carbon |
| Cu Ag Au Pt | Found in native state |
Note: Aluminium (Al) is the most abundant metal in the earth’s crust, iron (Fe) and calcium (Ca) being the second and third metals in the earth’s crust.
Minerals: The elements or compounds, which occur naturally in the earth’s crust, are known as minerals.
Ores: Those metals from which metals can be extracted profitably are called ores. Some examples of ores of different metals are:
| Types of iron ores | Common name and formula |
|---|---|
| Oxide ore | Haematite (Fe2O3), Magnetite (Fe3O4), Limonite (Fe2O3.3H2O) |
| Carbonate ore | Siderite (FeCO3) |
| Sulphide ore | Iron pyrites (FeS2) |
| Types of aluminium ores | Common name and formula |
|---|---|
| Oxide ore | Bauxite (Al2O3.2H2O), Corundum (Al2O3) |
| Fluoride ore | Cryolite (Na3AlF6) |
| Silicate ore | Feldspar (KAlSi3O8) |
| Types of copper ores | Common name and formula |
|---|---|
| Oxide ore | Cuprite (Cu2O) |
| Carbonate ore | Malachite CuCO3. Cu(OH)2 |
| Sulphide ore | Copper glance (Cu2S), Copper pyrite (CuFeS2) |
| Types of mercury ores | Common name and formula |
|---|---|
| Oxide ore | montroydite (HgO) |
| Selenide ore | Tiemannite HgSe |
| Sulphide ore | Cinnabar (HgS) |
| Types of zinc ores | Common name and formula |
|---|---|
| Oxide ore | Zincite (ZnO) |
| Carbonate ore | Calamine (ZnCO3) |
| Sulphide ore | Zinc blende (ZnS) |
Gaunge: The unwanted earthly material present in an ore is called gangue. Gangue may be acidic or basic in nature. The impurities must be removed from the ore prior to the extraction of the metal. The processes used for removing the gangue from the ore are based on the differences between the physical or chemical properties of the gangue and the ore.
Flux: A substance that during smelting combines with the earthly impurities present in the ore to form a fusible slag is called a flux. There are two types of fluxes. They are –
(i) Acidic flux, e.g., silica (SiO2)
(ii) Basic flux, e.g., lime (CaO), limestone (CaCO3)
Metallurgy (Extraction of metals)
The process of extracting pure metals from their ores is called metallurgy. It contains various steps:
Concentration of ore: The removal of gangue from an ore is called concentration of ore or dressing or ore. The processes used for removing the gangue from the ore are based on the differences between the physical or chemical properties of the gangue and the ore. For example,
(a) Hydraulic washing method – for difference densities of ore and gangue.
(b) Magnetic separation method – for magnetic property of either of gangue or of ore.
(c) Froth floatation method – for sulphide ore, etc.
Extraction of metals low in the activity series
Metals low in the activity series are very unreactive. The oxides of these metals can be reduced to metals by heating alone. For example,
(i) Cinnabar (HgS): It is an ore of mercury. When it is heated in air, it is first converted into mercuric oxide (HgO). Mercuric oxide is then reduced to mercury on further heating.
$$\mathrm{\underset{Cinnabar\ \ }{2HgS( s)}} \ +\underset{( from\ air)}{\mathrm{\underset{Oxygen}{3O_{2}( g)}} \ } \ \xrightarrow{heat} \ \mathrm{\underset{Mercury\ ( II) \ oxide}{2HgO( s)}} +\ \mathrm{\underset{Sulphur\ dioxide\ }{2SO_{2}( g)}}$$
$$\mathrm{\underset{Mercury\ ( II) \ oxide}{2HgO( s)}}\xrightarrow{heat} \ \mathrm{\underset{Mercury\ metal}{2Hg( l)}} +\ \mathrm{\underset{Oxygen\ }{O_{2}( g)}}$$
(ii) Copper glance (Cu2S): When it is heated in air, partially gets oxidised and then the oxidised product reacts with the remaining copper glance to give copper metal.
$$\mathrm{\underset{Copper\ glance\ \ }{2Cu_{2} S( s)}} \ +\underset{( from\ air)}{\mathrm{\underset{Oxygen}{3O_{2}( g)}} \ } \ \xrightarrow{heat} \ \mathrm{\underset{Copper\ oxide}{2Cu_{2} O( s)}} +\ \mathrm{\underset{Sulphur\ dioxide\ }{2SO_{2}( g)}}$$
$$\mathrm{\underset{Copper\ oxide}{2Cu_{2} O( s)}} +\mathrm{\underset{Copper\ glance\ \ }{Cu_{2} S( s)}} \ \xrightarrow{heat} \ \mathrm{\underset{Copper\ metal}{6Cu( s)}} +\ \mathrm{\underset{Sulphur\ dioxide\ }{SO_{2}( g)}}$$
Extracting Metals in the Middle of the Activity Series
The metals in the middle of the activity series such as iron, zinc, lead, copper, etc., are moderately reactive. These are usually present as sulphides or carbonates in nature. It is easier to obtain metal from its oxide, as compared to its sulphides and carbonates. Therefore, before reduction, the metal sulphides and carbonates must be converted into metal oxides.
(i) Roasting: The process in which sulphide ores are converted into oxides by heating strongly in the presence of excess air is known as roasting. For example,
$$\mathrm{\underset{Zinc\ sulphide\ }{2ZnS( s)}} \ +\underset{( from\ air)}{\mathrm{\underset{Oxygen}{3O_{2}( g)}} \ } \ \xrightarrow{heat} \ \mathrm{\underset{Zinc\ oxide}{2ZnO( s)}} +\ \mathrm{\underset{Sulphur\ dioxide\ }{2SO_{2}( g)}}$$
(ii) Calcination: The process in which carbonate ores are converted into oxides by heating strongly in limited air is known as calcination. For example,
$$\mathrm{\underset{Zinc\ carbonate\ \ }{ZnCO_{3}( s)}} \ \ \xrightarrow{heat} \ \ \ \mathrm{\underset{Zinc\ oxide}{ZnO( s)}} +\ \mathrm{\underset{Carbon\ dioxide\ }{CO_{2}( g)}}$$
(iii) Reduction of oxide ore: It is the process of conversion of metal oxide ore into metal. The metal oxides obtained by roasting and calcination are then reduced to the corresponding metals by using suitable reducing agents such as carbon. For example, when zinc oxide is heated with carbon, it is reduced to metallic zinc.
$$\mathrm{\underset{Zinc\ oxide\ }{ZnO( s)}} \ +\mathrm{\underset{Coke}{C( s)}} \ \ \ \xrightarrow{heat} \ \mathrm{\underset{Zinc\ metal}{\ \ Zn( s)}} +\ \mathrm{\underset{Carbon\ monoxide\ }{CO( g)}}$$
(iv) Displacement reaction: Sometime displacement reaction is used to reduce metal oxides into metals. The highly reactive metals such as sodium, calcium, aluminium, etc., are used as reducing agents because they can displace metals of lower reactivity from their compounds. These displacement reactions are highly exothermic. The amount of heat evolved is so large that the metals are produced in the molten state. For example,
(a) when manganese dioxide is heated with aluminium powder, the following reaction takes place –
$$\mathrm{\underset{Manganese\ oxide\ }{3MnO_{2}( s)}} \ +\mathrm{\underset{Aluminium}{4Al( s)}} \ \ \ \xrightarrow \ \mathrm{\underset{Manganese\ metal}{\ \ 3Mn( l)}} +\ \mathrm{\underset{Aluminium\ oxide\ }{2Al_{2} O_{3}( g)}} +\mathrm{\ heat}$$
(b) The reaction of iron(III) oxide (Fe2O3) with aluminium is used to join railway tracks or cracked machine parts. This process is called thermit welding.
$$\mathrm{\underset{Iron( III) \ oxide\ }{Fe_{2} O_{3}( s)}} \ +\mathrm{\underset{Aluminium}{2Al( s)}} \ \ \ \longrightarrow \ \mathrm{\underset{Iron\ ( molten)}{\ \ 2Fe( l)}} +\ \mathrm{\underset{Aluminium\ oxide\ }{Al_{2} O_{3}( g)}} +\mathrm{\ heat}$$
The reaction of metal oxide to form metal by using aluminium powder as a reducing agent is know as thermit reaction.

| Calcination | Roasting |
|---|---|
| 1. During calcination, the ore is heated in the presence of no or limited quantity of air. | 1. During roasting, the ore is heated in the presence of an excess of air. |
| 2. Calcination is generally used to convert carbonate ores into oxide ores. | 2. Roasting is generally used to convert sulphide ores into oxide ores. |
Extracting Metals towards the Top of the Activity Series
The metals at the top in the reactivity series are very reactive. They cannot be obtained from their compounds by heating with carbon i.e., carbon cannot reduce the oxides of sodium, magnesium, calcium, aluminium, etc., to the respective metals. This is because these metals have more affinity for oxygen than carbon.
These metals are obtained by electrolytic reduction. For example, sodium, magnesium, and calcium are obtained by the electrolysis of their molten chlorides. The metals are deposited at the cathode (the negatively charged electrode), whereas, chlorine is liberated at the anode (the positively charged electrode). The reactions are –
At cathode (reduction): Na+ + e– → Na
At anode (oxidation): 2Cl– → Cl2 + 2e–
Similarly, aluminium is obtained by the electrolytic reduction of aluminium oxide. Aluminium metal is obtained at the cathode, while oxygen gas is liberated at the anode.
At cathode (reduction): 2Al3+ + 6e– → 2Al
At anode (oxidation): 3O2– → 3/2O2 + 6e–
Refining of metals
The metals produced by various reduction processes are not very pure. They contain impurities, which must be removed to obtain pure metals.
The process of purification of the metal obtained after reduction is called refining of metals.
There are various methods used to refine impure metals. It depends on the nature of metal to be refined. The most widely used method for refining impure metals is electrolytic refining.
Electrolytic Refining
Many metals, such as copper, zinc, tin, nickel, silver, gold, etc., are refined electrolytically. We use this process to refine impure copper.
Construction: The apparatus for this process is setup as shown in the figure.

Working process: In this process, the impure metal (Cu) is made the anode and a thin strip of pure metal (Cu) is made the cathode. A solution of the metal salt (CuSO4) is used as an electrolyte. On passing the current through the electrolyte, the pure metal (Cu) from the anode dissolves into the electrolyte. An equivalent amount of pure metal (Cu) from the electrolyte is deposited on the cathode. The soluble impurities go into the solution, whereas, the insoluble impurities settle down at the bottom of the anode and are known as anode mud.
Corrosion
It is the slow process of eating away of metals by the reaction of atmospheric air and moisture.
In other words, The surface of some metals, such as iron, is corroded when they are exposed to moist air for a long period of time. This phenomenon is known as corrosion. For example,
(i) Silver articles become black after some time when exposed to air. This is because it reacts with sulphur in the air to form a coating of silver sulphide.
(ii) Copper reacts with moist carbon dioxide in the air and slowly loses its shiny brown surface and gains a green coat. This green substance is copper carbonate.
(iii) Iron when exposed to moist air for a long time acquires a coating of a brown flaky substance called rust.
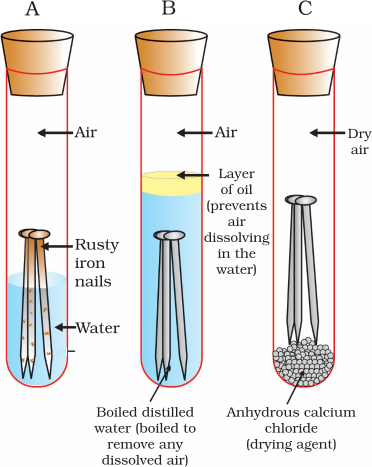
Testing of Conditions of Iron Rust
We will observe that iron nails rust in test tube A, but they do not rust in test tubes B and C. In the test tube A, the nails are exposed to both air and water. In the test tube B, the nails are exposed to only water, and the nails in test tube C are exposed to dry air.
Factors affecting the rate of corrosion
(i) Electropositive nature of the metal.
(ii) Purity of the metal.
(iii) Presence of reactive gases in the air.
(iv) Presence of electrolytes in water.
(v) Temperature
Prevention of Corrosion
The rusting of iron can be prevented by painting, oiling, greasing, galvanising, chrome plating, anodising, or making alloys.
(i) Galvanisation: It is a method of protecting steel and iron from rusting by coating them with a thin layer of zinc. It is done by dipping the article into molten zinc. The galvanised article is protected against rusting even if the zinc coating is broken.
(ii) Alloying: It is the method of improving the properties of a metal by mixing the metal with other metals or non-metals. The properties of metal change if it is mixed with some other substance. For example,
(a) Alloying of iron: Pure iron is very soft and stretches easily when hot. But, if it is mixed with a small amount of carbon (about 0.05 %), it becomes hard and strong. Iron is mixed with many metals to form different alloys. When iron is mixed with nickel and chromium, we get stainless steel, which is hard and does not rust. Thus, if iron is mixed with some other substance, its properties also change.
(b) Alloying of gold: Pure gold, known as 24-carat gold, is very soft. It is, therefore, not suitable for making jewellery. It is alloyed with either silver or copper to make it hard. Generally, in India, 22-carat gold is used for making ornaments. It means that 22 parts of pure gold are alloyed with 2 parts of either copper or silver.
(iii) Greasing or Oiling: When grease or oil is applied to the surface of an iron object then air and moisture cannot come in contact with it and hence, rust is prevented. For example, tools and machine parts made up of iron are smeared with grease.
(iv) Electroplating: Electroplating of iron with corrosion-resistance metals like tin, chromium, etc. resists it from corrosion. So, a thin layer of tin or chromium is deposited on the surface of an iron object by electroplating. Electroplating is the method of deposition of a layer of other metal on the surface of a metal by using electricity.
Alloy
An alloy is a homogeneous mixture of two or more metals, or a metal and a nonmetal.
It is prepared by first melting the primary metal, and then, dissolving the other elements in it in definite proportions. It is then cooled to room temperature. For example,
(i) Amalgam: If one of the metals is mercury, then the alloy is known as an amalgam. For example, zinc amalgam (Zn-Hg), sodium amalgam (Na-Hg), Dental amalgam is a mixture of metals, consisting of liquid (elemental) mercury and a powdered alloy composed of silver, tin, and copper. etc.
(ii) Brass: an alloy of copper and zinc (Cu and Zn).
(iii) Bronze: an alloy of copper and tin (Cu and Sn).
(iv) Stainless steel: an alloy of iron, nickel and chromium ( Fe, Ni, and Cr)
(v) Solder: an alloy of lead and tin (Pb and Sn).
Properties of alloys:
(i) Alloys do not get corroded or get corroded to very less extent.
(ii) They are harder and stronger than pure metals e.g., gold is mixed with copper and it is harder than pure gold.
(iii) They have less conductance than pure metals e.g., copper is good conductor of heat and electricity whereas brass and bronze are not good conductors.
(iv) Some alloys have lower melting point than pure metals e.g., solder is an alloy of lead and tin which has lower melting point than each of the metals.
The wonder of ancient Indian metallurgy
The iron pillar near the Qutub Minar in Delhi was made around 400 BC by the iron workers of India. They had developed a process which prevented wrought iron from rusting. This is likely because of formation of a thin film of magnetic oxide (Fe3O4) on the surface, as a result of finishing treatment given to the pillar, painting it with a mixture of different salts, then heating and quenching. The iron pillar is 8 m high and weighs 6 tonnes (6000 kg).

Questions
Q.1. Why is sodium kept immersed in kerosene oil?
Ans: Sodium metal being reactive highly reacts so vigorously with oxygen that it catches fire if kept in the open air. Therefore, to protect it from accidental fires, sodium is kept immersed in kerosene oil.
Q.2. Why do ionic compounds have high melting points?
Ans: In ionic compounds, strong electrostatic forces of attraction are present between the appositively charged ions. When these compounds are heated, a lot of energy is consumed to break these strong electrostatic forces of attraction during melting. Therefore, ionic compounds have high melting points.
Q.3. Which metals do not corrode easily?
Ans: Metals present at the bottom of the reactivity series do not corrode easily, e.g., gold, silver, platinum, etc.
Q.4. Platinum, gold, and silver are used to make jewellery. Give reason.
Ans: These elements are highly malleable, lustrous, and least reactive. So, they are not corroded by air and water easily.
Q.5. Aluminium is a highly reactive metal, yet it is used to make utensils for cooking. Give reason.
Ans: Due to the formation of a thin layer of aluminium oxide on the surface of aluminium, it is prevented from corrosion. Thus, aluminium vessels do not react with any ingredient of food and are suitable for cooking.
Q.6. Carbonate and sulphide ores are usually converted into oxides during the process of extraction. Give reason.
Ans: Metals can be easily extracted from their oxides rather than sulphides and carbonates. Therefore, these ores are first converted to oxides.
Q.7. Why copper is used to make hot water tanks and not steel (an alloy of iron)?
Ans: Iron (or steel) is more reactive than copper when in contact with steam formed from hot water. So, the body of tank is made of copper but not steel as copper does not react with water.
Previous Year Questions
Short Questions
Q.1. What is meant by electrolytic reduction? How is sodium obtained from its molten chloride? Explain [2010]
Q.2. Explain the reactions of different metals with hot water, cold water, and steam. Give one example with a proper balanced chemical equation. Name two metals which do not react with any form of water. [2012]
Q.3. State the property utilised in the following:
(i) Graphite in making electrodes.
(ii) Electrical wires are coated with Polyvinyl Chloride (PVC) or a rubber-like material.
(iii) Metal alloys are used for making bells and strings of musical instruments. [2012]
Q.4. P, Q and R are 3 elements which undergo chemical reactions according to the following equations:
(a) P2O3 + 2Q → Q2O3 + 2P
(b) 3 RSO4 + 2Q → Q2(SO4)3 + 3R
(c) 3RO + 2P → P2O3 + 3R
Answer the following questions:
(i) Which element is most reactive?
(ii) Which element is least reactive?
(iii) State the type of reaction listed above. [2014]
Q.5. (i) Write any two properties of ionic compounds;
(ii) Show the formation of aluminium chloride by the transfer of electrons between the atoms. (Atomic number of aluminium and chlorine are 13 and 17 respectively). [2015]
Q.6. Account for the following:
(i) Electrical wires are coated with plastic.
(ii) Carbon is not used for reducing aluminium from aluminium oxide. [2016]
Q.7. Explain the process of electrolytic refining for copper with the help of a labelled diagram. [2016]
Q.8. A metal X, which is used in thermite process, when heated with oxygen gives an oxide Y which is amphoteric in nature. Identify X and Y. Write balanced chemical equations of the reactions of oxide Y with hydrochloric acid and sodium hydroxide. [2019]
Q.9. How is the method of extraction of metals high up in the reactivity series different from that for metals in the middle? Why can be same process not be applied for them? Name the process used for the extraction of these metals. [2019]
Q.10. (a) By the transfer of electrons, illustrate the formation of bond in magnesium chloride and identify the ions present in this compound.
(b) Ionic compounds are solids. Give reason.
(c) With the help of labelled diagram show the experimental set up of action of steam of a metal. [2020]
Q.11. A reddish brown metal used in electrical wires when powdered and heated strongly turns black. When hydrogen gas is passed over this black substance, it regains its original colour. Based on this information answer the following questions-
(a) Name the metal and the black substance formed.
(b) Write balanced chemical equations for the two reactions involved in the above information. [2023]
Long Questions
Q.1. In what forms are metals found in nature? With the help of examples, explain how metals react with oxygen, water, and dilute acids. Also, write chemical equations for the reactions. [2010,11]
Q.2. Give reasons for the following:
(i) Ionic compounds have higher melting and boiling points.
(ii) Sodium is kept immersed in kerosene.
(iii) Reaction of calcium with water is less violent.
(iv) Prior to reduction the metal sulphides and carbonates must be converted into metal oxides for extracting metals. [2014]
Q.3. Give reasons for the following:
(i) Generally no hydrogen gas is evolved when metals react with dilute nitric acid.
(ii) Sodium hydroxide solution cannot be kept in aluminium containers.
(iii) Silver metal does not combine easily with oxygen but silver jewellery tarnishes after some time.
(iv) Sodium is obtained by the electrolysis of its molten chloride and not from its aqueous solution.
(v) Aluminium reacts with dilute hydrochloric acid slowly in the begining. [2014]
Q.4. How is copper obtained from its ore (Cu2S)? Write only the chemical equations. How is copper thus obtained refined? Name and explain the process along with a labelled diagram. [2014]
Q.5. (i) Write the steps involved in the extraction of pure metals in the middle of the activity series from their carbonate ores.
(ii) How is copper extracted from its sulphide ore? Explain the various steps supported by chemical equations. Draw labelled diagram for the electrolytic refining of copper. [2018]
Q.6. (i) Write chemical equations for the following reactions:
(a) Calcium metal reacts with water.
(b) Cinnabar is heated in the presence of air.
(c) Magnesium dioxide is heated with aluminium powder. [2019]
Q.7. Explain the following:
(i) Sodium chloride is an ionic compound which does not conduct electricity in solid state whereas it does conduct electricity in molten state as well as in aqueous solution.
(ii) Reactivity of aluminium decrease if it is dipped in nitric acid.
(iii) Metals like calcium and magnesium are never found in their free state in nature. [2019]
Q.8. Two ores X and Y were taken. On heating these ores, it was observed that
(a) ore X gives CO2 gas, and
(b) ore Y gives SO2 gas.
Write steps to convert these ores into metals, giving chemical equations of the reactions that take place. [2020]
Q.9. (a) Write the help of a diagram explain the method of refining of copper by electrolysis.
(b) How are broken railway tracks joined? Give the name of the process and the chemical equation of the reaction involved. [2020]
Case Study Based Questions
Q.1. On the basis of reactivity metals are grouped into three categories –
(i) Metals of low reactivity
(ii) Metals of medium reactivity
(iii) Metals of high reactivity
Therefore metals are extracted in pure form from their ores on the basis of their chemical properties.
Metals of high reactivity are extracted from their ores by electrolysis of the molten ore.
Metals of low reactivity are extracted from their sulphide ores, which are converted into their oxides. The oxides of these metals are reduced to metals by simple heating.
(a) Name the process of reduction used for a metal that gives vigorous reaction with air and water both.
(b) Carbon cannot be used as a reducing agent to obtain aluminium from its oxide? Why?
(c) Describe briefly the method to obtain mercury from cinnabar. Write the chemical equation for the reactions involved in the process.
or,
(c) Differentiate between roasting and calcination giving chemical equation of each. [2023]
