It is the tendency of an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. It is denoted by a Greek alphabet chi ‘χ‘,
Characteristics of electronegativity
1. Electronegativity measures the power of attracting electrons. The higher the associated electronegativity, the more an atom or a substituent group attracts electrons.
2. Electronegativity estimates the bond energy quantitatively, and the sign and magnitude of a bond’s chemical polarity, which characterizes a bond along the continuous scale from covalent to ionic bonding.
3. Electronegativity determines the nature of bonds.
(a) If χA –χB = 0, the bond is purely covalent and non-polar. For example, H2, Cl2, O2, N2, etc.
(b) If χA –χB < 1.9, the bond is polar covalent (more covalent less ionic).
(c) If χA –χB = 1.9, the bond is 50% ionic and 50% covalent.
(d) If χA –χB > 1.9, the bond is more ionic less covalent.
4. Percentage of ionic character = 16(χA –χB)+3.5(χA –χB)2, where χA and χB represent electronegativity of bonded atoms A and B.
This relation is given by Allred in 1961.
5. Electronegativity determines the nature of oxides.
(a If χO –χA is large, the oxide shows basic nature (e.g., Na2O, MgO, etc.).
(b) If χO –χA is small, the oxide shows acidic nature (e.g., SO2, CO2, etc.).
Determination of electronegativity
Electronegativity is determined by two factors mainly i.e., nuclear charge and the number and location of other electrons in the atomic shells.
1. Nuclear charge: The more protons an atom has, the more “pull” it will have on electrons.
2. The number and location of other electrons in the atomic shells: The more electrons an atom has, the farther from the nucleus the valence electrons will be, and as a result, the less positive charge they will experience—both because of their increased distance from the nucleus and because the other electrons in the lower energy core orbitals will act to shield the valence electrons from the positively charged nucleus.
Calculation of electronegativity
Electronegativity cannot be directly measured and must be calculated from other atomic or molecular properties. Several methods of calculation have been proposed, and although there may be small differences in the numerical values of electronegativity, all methods show the same periodic trends between elements.
1. Pauling’s scale: Pauling first proposed the concept of electronegativity in 1932 to explain why the covalent bond between two different atoms (A–B) is stronger than the average of the A–A and the B–B bonds. The difference in electronegativity between atoms A and B is given by
$$\chi_A-\chi_B=0.208\left[E_{\left(A-B\right)}-\sqrt{\left(E_{\left(A-A\right)}-E_{\left(B-B\right)}\right)}\right]^{\left(1/2\right)}$$
where χA and χB are the electronegativity of two atoms A and B and EA-B, EA-A and EB-B are bond energies of molecules A-B, A2 and B2, respectively in kcal mol-1.
If the bond energies are taken in kj mol-1, then
$$\chi_A-\chi_B=0.102\left[E_{\left(A-B\right)}-\sqrt{\left(E_{\left(A-A\right)}-E_{\left(B-B\right)}\right)}\right]^{\left(1/2\right)}$$
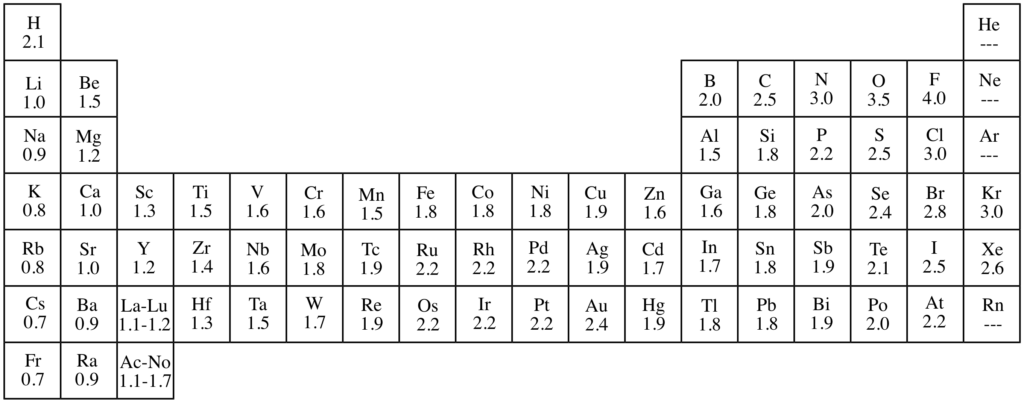
The range of values for Pauling’s scale of electronegativity ranges from Fluorine (most electronegative = 4.0) to Francium (least electronegative = 0.7).
2. Mulliken’s scale: Robert S. Mulliken proposed that the arithmetic mean of the first ionization energy (Eie) and the electron affinity (Eea) should be a measure of the tendency of an atom to attract electrons i.e., the electronegativity. If the values are taken in eV then, electronegativity is
$$\chi=\frac{E_{ie}+E_{ea}}{2}$$
However, it was found that Mulliken values were 2.8 times greater than Pauling values. Thus, in the Pauling scale, electronegativity is
$$\chi=\frac{E_{ie}+E_{ea}}{2×2.8}=\frac{E_{ie}+E_{ea}}{5.6}$$
If the values are taken in kcal mol-1, then Pauling electronegativity is
$$\chi=\frac{E_{ie}+E_{ea}}{2×2.8×23.06}=\frac{E_{ie}+E_{ea}}{129}$$
If the values are taken in kJ mol-1, then Pauling electronegativity is
$$\chi=\frac{E_{ie}+E_{ea}}{2×2.8×96.48}=\frac{E_{ie}+E_{ea}}{540}$$
3. Allred and Rochow scale: A. Louis Allred and Eugene G. Rochow considered that electronegativity should be related to the charge experienced by an electron on the “surface” of an atom: The higher the charge per unit area of atomic surface the greater the tendency of that atom to attract electrons. The effective nuclear charge, Zeff, experienced by valence electrons can be estimated using Slater’s rules, while the surface area of an atom in a molecule can be taken to be proportional to the square of the covalent radius, rcov. When rcov is expressed in angstrom, then
$$\chi=0.744+\frac{0.359Z_{eff}}{r^2_{cov}}$$
Allred and Rochow’s method depends on measuring covalent radius and these are obtained with great accuaracy by X-ray crystallography so it might be expected to yield very accurate electronegativity values.
Factors affecting electronegativity
The electronegativity of any given element is not constant but depends on the following factors:
1. Size of atoms: Smaller the size of atom, greater is the attraction of electrons and hence, greater is the electronegativity.
2. State of hybridization: Electronegativity decreases with decrease in s character so it decreases in order: sp > sp2 > sp3.
| Hybridization | χ(pauling) |
|---|---|
| C (SP3) – 25 % S – Character | 2.3 |
| C (SP2) – 33.3 % S – Character | 2.6 |
| C (SP) – 50% S – Character | 3.1 |
| Generic ‘C’ | 2.5 |
3. Oxidation state of the element: With increase in oxidation state, electronegativity increases. For example, Fe 3+ (χ = 1.96) has higher electronegativity than Fe2+ (χ = 1.83).
Periodic Trends
In a period from left to right, the value of electronegativity increases.
In a group from top to bottom, the value of electronegativity decreases.
Note: The elements having low values of electronegativity are metals while the elements having high values of electronegativity are non-metals. Fluorine (F) with highest electronegativity is the most non-metallic and Caesium with lowest electronegativity is the most metallic element of the periodic table.
