Ethanol (C2H5OH) and phenol (C6H5OH) may be distinguished by the following four tests:
(i) Litmus Test: Phenol being acidic turns blue litmus red while ethanol being neutral does not give this test.
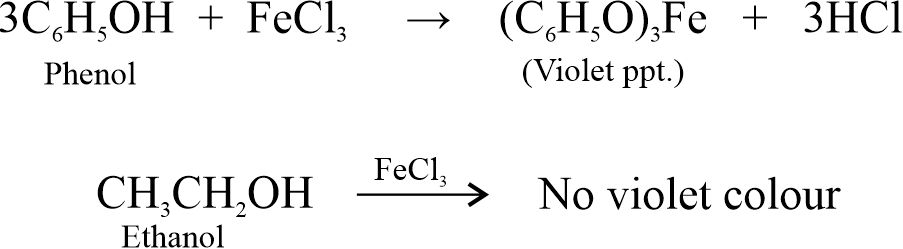
(ii) FeCl3 Test: Phenol gives a violet colour with FeCl3 solution while ethanol does not.
(iii) Iodoform Test: Ethanol on warming with NaOI (I2/NaOH) gives yellow ppt. of iodoform while phenol does not give this test.

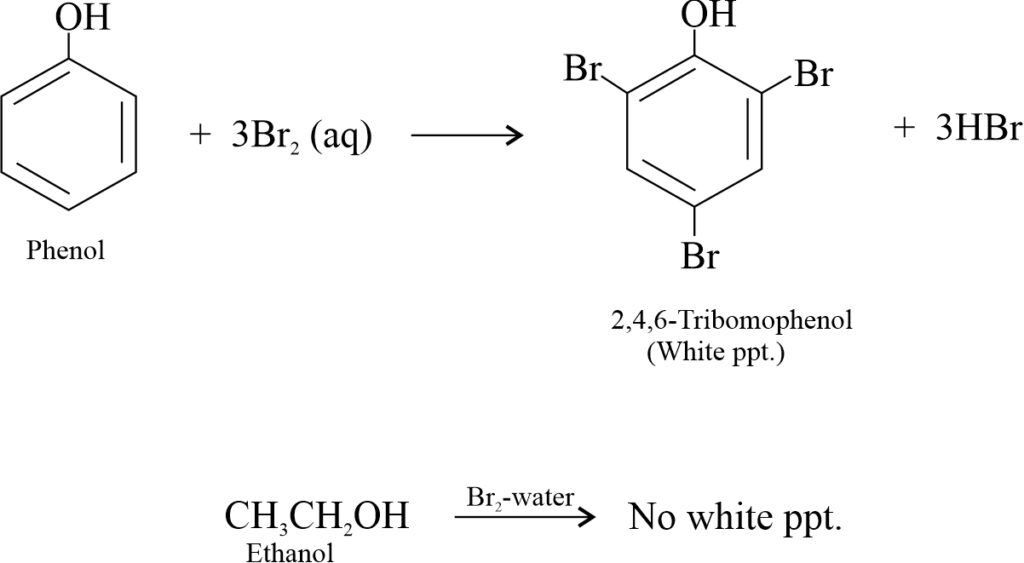
(iv) Bromine-water test: Phenol readily undergoes electrophilic substitution reactions. Therefore, it decolourises bromine water giving white ppt. of 2,4,6-tribromophenol. Ethanol does not give this test.