Formic acid (HCOOH) contains both an aldehydic (-CHO) as well as a carboxyl group (-COOH) but acetic acid (CH3COOH) contains only a carboxyl group. Therefore, formic acid behaves as a reducing agent whereas acetic acid does not. These acids may be distinguished by the following tests:
(i) Fehling’s solution test: Formic acid reduces Fehling’s solution to red ppt. of Cu2O but acetic acid does not.

(ii) Tollens’ reagent test: Formic acid reduces Tollens’ reagent to metallic silver but acetic acid does not.
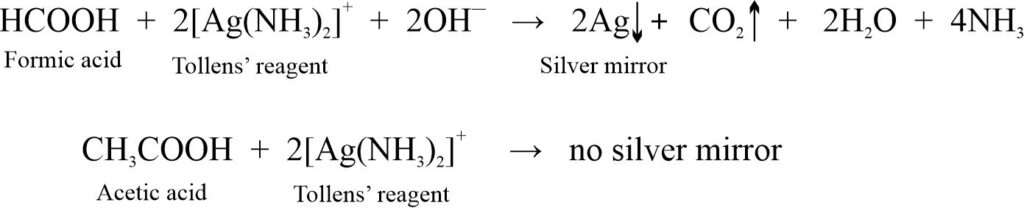
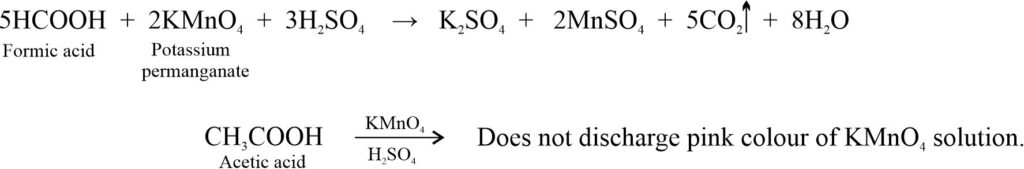
(iii) KMnO4 test: Formic acid decolourises acidified KMnO4 solution while acetic acid does not give this test.
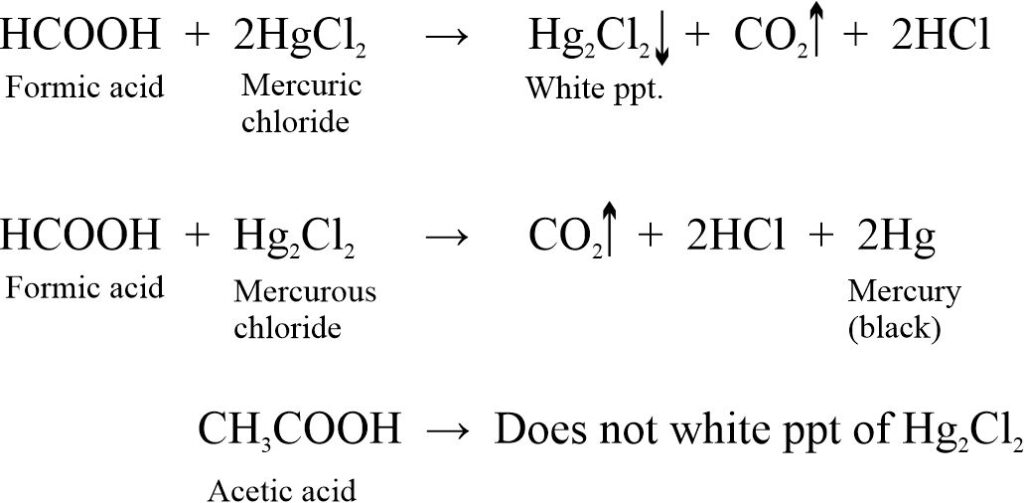
(iv) HgCl2 test: Formic acid reduces HgCl2 to give white ppt. of Hg2Cl2 which changes to mercury black while acetic acid does not give this test.